Protein thermostability above 100 degreesC: a key role for ionic interactions
- PMID: 9770481
- PMCID: PMC22826
- DOI: 10.1073/pnas.95.21.12300
Protein thermostability above 100 degreesC: a key role for ionic interactions
Abstract
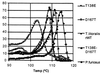
The discovery of hyperthermophilic microorganisms and the analysis of hyperthermostable enzymes has established the fact that multisubunit enzymes can survive for prolonged periods at temperatures above 100 degreesC. We have carried out homology-based modeling and direct structure comparison on the hexameric glutamate dehydrogenases from the hyperthermophiles Pyrococcus furiosus and Thermococcus litoralis whose optimal growth temperatures are 100 degreesC and 88 degreesC, respectively, to determine key stabilizing features. These enzymes, which are 87% homologous, differ 16-fold in thermal stability at 104 degreesC. We observed that an intersubunit ion-pair network was substantially reduced in the less stable enzyme from T. litoralis, and two residues were then altered to restore these interactions. The single mutations both had adverse effects on the thermostability of the protein. However, with both mutations in place, we observed a fourfold improvement of stability at 104 degreesC over the wild-type enzyme. The catalytic properties of the enzymes were unaffected by the mutations. These results suggest that extensive ion-pair networks may provide a general strategy for manipulating enzyme thermostability of multisubunit enzymes. However, this study emphasizes the importance of the exact local environment of a residue in determining its effects on stability.
Figures


References
-
- Adams M W. Annu Rev Microbiol. 1993;47:627–658. - PubMed
-
- Chan M K, Mukund S, Kletzin A, Adams M W, Rees D C. Science. 1995;267:1463–1469. - PubMed
-
- Hurley J H, Baase W A, Matthews B W. J Mol Biol. 1992;224:1143–1159. - PubMed
-
- Britton K L, Baker P J, Borges K M, Engel P C, Pasquo A, Rice D W, Robb F T, Scandurra R, Stillman T J, Yip K S P. Eur J Biochem. 1995;229:688–695. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

