Normal hepatic glucose production in the absence of GLUT2 reveals an alternative pathway for glucose release from hepatocytes
- PMID: 9770484
- PMCID: PMC22829
- DOI: 10.1073/pnas.95.21.12317
Normal hepatic glucose production in the absence of GLUT2 reveals an alternative pathway for glucose release from hepatocytes
Abstract
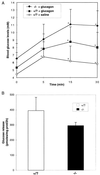
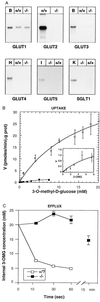
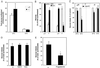
Glucose production by liver is a major physiological function, which is required to prevent development of hypoglycemia in the postprandial and fasted states. The mechanism of glucose release from hepatocytes has not been studied in detail but was assumed instead to depend on facilitated diffusion through the glucose transporter GLUT2. Here, we demonstrate that in the absence of GLUT2 no other transporter isoforms were overexpressed in liver and only marginally significant facilitated diffusion across the hepatocyte plasma membrane was detectable. However, the rate of hepatic glucose output was normal. This was evidenced by (i) the hyperglycemic response to i.p. glucagon injection; (ii) the in vivo measurement of glucose turnover rate; and (iii) the rate of release of neosynthesized glucose from isolated hepatocytes. These observations therefore indicated the existence of an alternative pathway for hepatic glucose output. Using a [14C]-pyruvate pulse-labeling protocol to quantitate neosynthesis and release of [14C]glucose, we demonstrated that this pathway was sensitive to low temperature (12 degreesC). It was not inhibited by cytochalasin B nor by the intracellular traffic inhibitors brefeldin A and monensin but was blocked by progesterone, an inhibitor of cholesterol and caveolae traffic from the endoplasmic reticulum to the plasma membrane. Our observations thus demonstrate that hepatic glucose release does not require the presence of GLUT2 nor of any plasma membrane glucose facilitative diffusion mechanism. This implies the existence of an as yet unsuspected pathway for glucose release that may be based on a membrane traffic mechanism.
Figures
References
-
- DeFronzo R A. Diabetes. 1988;37:667–687. - PubMed
-
- DeFronzo R A, Bonadonna R C, Ferrannini E. Diabetes Care. 1992;15:318–368. - PubMed
-
- Pilkis S J, el-Maghrabi M R, Claus T H. Annu Rev Biochem. 1988;57:755–783. - PubMed
-
- Pilkis S J, Granner D K. Annu Rev Physiol. 1992;54:885–909. - PubMed
-
- Burchell A. FASEB J. 1990;4:2978–2988. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

