GIPC, a PDZ domain containing protein, interacts specifically with the C terminus of RGS-GAIP
- PMID: 9770488
- PMCID: PMC22833
- DOI: 10.1073/pnas.95.21.12340
GIPC, a PDZ domain containing protein, interacts specifically with the C terminus of RGS-GAIP
Abstract
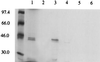
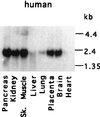
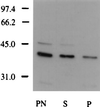
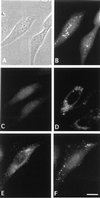
We have identified a mammalian protein called GIPC (for GAIP interacting protein, C terminus), which has a central PDZ domain and a C-terminal acyl carrier protein (ACP) domain. The PDZ domain of GIPC specifically interacts with RGS-GAIP, a GTPase-activating protein (GAP) for Galphai subunits recently localized on clathrin-coated vesicles. Analysis of deletion mutants indicated that the PDZ domain of GIPC specifically interacts with the C terminus of GAIP (11 amino acids) in the yeast two-hybrid system and glutathione S-transferase (GST)-GIPC pull-down assays, but GIPC does not interact with other members of the RGS (regulators of G protein signaling) family tested. This finding is in keeping with the fact that the C terminus of GAIP is unique and possesses a modified C-terminal PDZ-binding motif (SEA). By immunoblotting of membrane fractions prepared from HeLa cells, we found that there are two pools of GIPC-a soluble or cytosolic pool (70%) and a membrane-associated pool (30%). By immunofluorescence, endogenous and GFP-tagged GIPC show both a diffuse and punctate cytoplasmic distribution in HeLa cells reflecting, respectively, the existence of soluble and membrane-associated pools. By immunoelectron microscopy the membrane pool of GIPC is associated with clusters of vesicles located near the plasma membrane. These data provide direct evidence that the C terminus of a RGS protein is involved in interactions specific for a given RGS protein and implicates GAIP in regulation of additional functions besides its GAP activity. The location of GIPC together with its binding to GAIP suggest that GAIP and GIPC may be components of a G protein-coupled signaling complex involved in the regulation of vesicular trafficking. The presence of an ACP domain suggests a putative function for GIPC in the acylation of vesicle-bound proteins.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

