Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Calpha
- PMID: 9770493
- PMCID: PMC22838
- DOI: 10.1073/pnas.95.21.12370
Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Calpha
Abstract
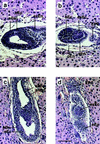
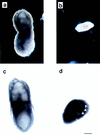
Protein phosphatase 2A (PP2A) is a multimeric enzyme, containing a catalytic subunit complexed with two regulatory subunits. The catalytic subunit PP2A C is encoded by two distinct and unlinked genes, termed Calpha and Cbeta. The specific function of these two catalytic subunits is unknown. To address the possible redundancy between PP2A and related phosphatases as well as between Calpha and Cbeta, the Calpha subunit gene was deleted by homologous recombination. Homozygous null mutant mice are embryonically lethal, demonstrating that the Calpha subunit gene is an essential gene. As PP2A exerts a range of cellular functions including cell cycle regulation and cell fate determination, we were surprised to find that these embryos develop normally until postimplantation, around embryonic day 5.5/6.0. While no Calpha protein is expressed, we find comparable expression levels of PP2A C at a time when the embryo is degenerating. Despite a 97% amino acid identity, Cbeta cannot completely compensate for the absence of Calpha. Degenerated embryos can be recovered even at embryonic day 13.5, indicating that although embryonic tissue is still capable of proliferating, normal differentiation is significantly impaired. While the primary germ layers ectoderm and endoderm are formed, mesoderm is not formed in degenerating embryos.
Figures



References
-
- Snaith H A, Armstrong C G, Guo Y, Kaiser K, Cohen P T W. J Cell Sci. 1996;109:3001–3012. - PubMed
-
- Cohen P. Annu Rev Biochem. 1989;58:453–508. - PubMed
-
- Shenolikar S, Nairn A C. In: Advances in Second Messengers and Phosphoprotein Research. Greengard P, Robinson G A, editors. Vol. 25. New York: Raven; 1991. pp. 1–121. - PubMed
-
- Khew-Goodall Y, Hemmings B. FEBS Lett. 1988;238:265–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

