Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant
- PMID: 9770502
- PMCID: PMC22847
- DOI: 10.1073/pnas.95.21.12424
Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant
Abstract
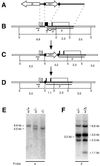
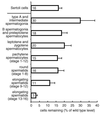
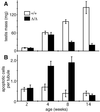
Proteins of the Bcl-2 family are important regulators of apoptosis in many tissues of the embryo and adult. The recently isolated bcl-w gene encodes a pro-survival member of the Bcl-2 family, which is widely expressed. To explore its physiological role, we have inactivated the bcl-w gene in the mouse by homologous recombination. Mice that lack Bcl-w were viable, healthy, and normal in appearance. Most tissues exhibited typical histology, and hematopoiesis was unaffected, presumably due to redundant function with other pro-survival family members. Although female reproductive function was normal, the males were infertile. The testes developed normally, and the initial, prepubertal wave of spermatogenesis was largely unaffected. The seminiferous tubules of adult males, however, were disorganized, contained numerous apoptotic cells, and produced no mature sperm. Both Sertoli cells and germ cells of all types were reduced in number, the most mature germ cells being the most severely depleted. The bcl-w-/- mouse provides a unique model of failed spermatogenesis in the adult that may be relevant to some cases of human male sterility.
Figures



References
-
- Strasser A, Huang D C S, Vaux D L. Biochim Biophys Acta. 1997;1333:F151–F178. - PubMed
-
- Adams, J. M. & Cory, S. (1998) Science, in press.
-
- Chao D T, Korsmeyer S J. Annu Rev Immunol. 1998;16:395–419. - PubMed
-
- Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. - PubMed
-
- Pan G H, O’Rourke K, Dixit V M. J Biol Chem. 1998;273:5841–5845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

