Kinetics of T cell receptor beta, gamma, and delta rearrangements during adult thymic development: T cell receptor rearrangements are present in CD44(+)CD25(+) Pro-T thymocytes
- PMID: 9770518
- PMCID: PMC22863
- DOI: 10.1073/pnas.95.21.12522
Kinetics of T cell receptor beta, gamma, and delta rearrangements during adult thymic development: T cell receptor rearrangements are present in CD44(+)CD25(+) Pro-T thymocytes
Abstract
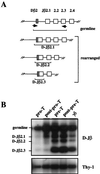
We performed a comprehensive analysis of T cell receptor (TCR) gamma rearrangements in T cell precursors of the mouse adult thymus. Using a sensitive quantitative PCR method, we show that TCRgamma rearrangements are present in CD44(+)CD25(+) Pro-T thymocytes much earlier than expected. TCRgamma rearrangements increase significantly from the Pro-T to the CD44(-)CD25(+) Pre-T cell transition, and follow different patterns depending on each Vgamma gene segment, suggesting that ordered waves of TCRgamma rearrangement exist in the adult mouse thymus as has been described in the fetal mouse thymus. Recombinations of TCRgamma genes occur concurrently with TCRdelta and D-Jbeta rearrangements, but before Vbeta gene assembly. Productive TCRgamma rearrangements do not increase significantly before the Pre-T cell stage and are depleted in CD4(+)CD8(+) double-positive cells from normal mice. In contrast, double-positive thymocytes from TCRdelta-/- mice display random proportions of TCRgamma rearranged alleles, supporting a role for functional TCRgamma/delta rearrangements in the gammadelta divergence process.
Figures

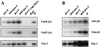
References
-
- Pardoll D M, Fowlkes B J, Bluestone J A, Kruisbeek A, Maloy W L, Coligan J E, Schwartz R H. Nature (London) 1987;326:79–81. - PubMed
-
- Raulet D H, Spencer D M, Hsiang Y H, Goldman J P, Bix M, Liao N S, Zijstra M, Jaenisch R, Correa I. Immunol Rev. 1991;120:185–204. - PubMed
-
- Haas W, Pereira P, Tonegawa S. Annu Rev Immunol. 1993;11:637–685. - PubMed
-
- Godfrey D I, Zlotnik A. Immunol Today. 1993;14:547–553. - PubMed
-
- Shortman K, Wu L. Annu Rev Immunol. 1996;14:29–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

