Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae
- PMID: 9770539
- PMCID: PMC22884
- DOI: 10.1073/pnas.95.21.12641
Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae
Abstract
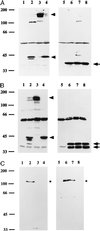
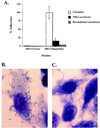
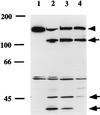
Haemophilus influenzae is a major cause of otitis media and other respiratory tract disease in children. The pathogenesis of disease begins with colonization of the upper respiratory mucosa, a process that involves evasion of local immune mechanisms and adherence to epithelial cells. Several studies have demonstrated that human milk is protective against H. influenzae colonization and disease. In the present study, we examined the effect of human milk on the H. influenzae IgA1 protease and Hap adhesin, two autotransported proteins that are presumed to facilitate colonization. Our results demonstrated that human milk lactoferrin efficiently extracted the IgA1 protease preprotein from the bacterial outer membrane. In addition, lactoferrin specifically degraded the Hap adhesin and abolished Hap-mediated adherence. Extraction of IgA1 protease and degradation of Hap were localized to the N-lobe of the bilobed lactoferrin molecule and were inhibited by serine protease inhibitors, suggesting that the lactoferrin N-lobe may contain serine protease activity. Additional experiments revealed no effect of lactoferrin on the H. influenzae P2, P5, and P6 outer-membrane proteins, which are distinguished from IgA1 protease and Hap by the lack of an N-terminal passenger domain or an extracellular linker region. These results suggest that human milk lactoferrin may attenuate the pathogenic potential of H. influenzae by selectively inactivating IgA1 protease and Hap, thereby interfering with colonization. Future studies should examine the therapeutic potential of lactoferrin, perhaps as a supplement in infant formulas.
Figures


Similar articles
-
Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites.Mol Microbiol. 2003 Feb;47(3):607-17. doi: 10.1046/j.1365-2958.2003.03327.x. Mol Microbiol. 2003. PMID: 12535064
-
Human lactoferrin proteolytic activity: analysis of the cleaved region in the IgA protease of Haemophilus influenzae.Vaccine. 2000 Dec 8;19 Suppl 1:S148-52. doi: 10.1016/s0264-410x(00)00296-6. Vaccine. 2000. PMID: 11163480
-
Inactivation of Haemophilus influenzae lipopolysaccharide biosynthesis genes interferes with outer membrane localization of the hap autotransporter.J Bacteriol. 2012 Apr;194(7):1815-22. doi: 10.1128/JB.06316-11. Epub 2012 Jan 27. J Bacteriol. 2012. PMID: 22287523 Free PMC article.
-
Structure and function of the Haemophilus influenzae autotransporters.Front Cell Infect Microbiol. 2011 Sep 28;1:5. doi: 10.3389/fcimb.2011.00005. eCollection 2011. Front Cell Infect Microbiol. 2011. PMID: 22919571 Free PMC article. Review.
-
Molecular determinants of the pathogenesis of disease due to non-typable Haemophilus influenzae.FEMS Microbiol Rev. 1999 Apr;23(2):99-129. doi: 10.1111/j.1574-6976.1999.tb00393.x. FEMS Microbiol Rev. 1999. PMID: 10234841 Review.
Cited by
-
Lactoferrin impairs pathogen virulence through its proteolytic activity.Front Vet Sci. 2024 Aug 8;11:1428156. doi: 10.3389/fvets.2024.1428156. eCollection 2024. Front Vet Sci. 2024. PMID: 39176399 Free PMC article. Review.
-
Inhibitory Effects of Synthetic Peptides Containing Bovine Lactoferrin C-lobe Sequence on Bacterial Growth.Korean J Food Sci Anim Resour. 2016;36(4):452-7. doi: 10.5851/kosfa.2016.36.4.452. Epub 2016 Aug 30. Korean J Food Sci Anim Resour. 2016. PMID: 27621684 Free PMC article.
-
Virus-neutralizing monoclonal antibody expressed in milk of transgenic mice provides full protection against virus-induced encephalitis.J Virol. 2001 Mar;75(6):2803-9. doi: 10.1128/JVI.75.6.2803-2809.2001. J Virol. 2001. PMID: 11222704 Free PMC article.
-
Bovine apo-lactoferrin affects the secretion of proteases in Mannheimia haemolytica A2.Access Microbiol. 2021 Oct 25;3(10):000269. doi: 10.1099/acmi.0.000269. eCollection 2021. Access Microbiol. 2021. PMID: 34816089 Free PMC article.
-
Analysis of nontypeable haemophilus influenzae phase-variable genes during experimental human nasopharyngeal colonization.J Infect Dis. 2013 Sep 1;208(5):720-7. doi: 10.1093/infdis/jit240. Epub 2013 May 28. J Infect Dis. 2013. PMID: 23715658 Free PMC article.
References
-
- Klein J O. Clin Infect Dis. 1994;19:823–833. - PubMed
-
- Teele D W, Klein J O, Rosner B the Greater Boston Otitis Media Study Group. J Infect Dis. 1989;160:83–94. - PubMed
-
- Teele D W, Klein J O, Rosner B the Greater Boston Otitis Media Study Group. J Am Med Assoc. 1983;249:1026–1029. - PubMed
-
- Fria T J, Cantekin E I, Eichler J A. Arch Otolaryngol Head Neck Surg. 1985;111:10–16. - PubMed
-
- Teele D W, Klein J O, Chase C, Menyuk P, Rosner B A the Greater Boston Otitis Media Study Group. J Infect Dis. 1990;162:685–694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

