Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice
- PMID: 9770546
- PMCID: PMC22891
- DOI: 10.1073/pnas.95.21.12683
Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice
Abstract
When administered intracerebroventricularly to mice performing various learning tasks involving either short-term or long-term memory, secreted forms of the beta-amyloid precursor protein (APPs751 and APPs695) have potent memory-enhancing effects and block learning deficits induced by scopolamine. The memory-enhancing effects of APPs were observed over a wide range of extremely low doses (0.05-5,000 pg intracerebroventricularly), blocked by anti-APPs antisera, and observed when APPs was administered either after the first training session in a visual discrimination or a lever-press learning task or before the acquisition trial in an object recognition task. APPs had no effect on motor performance or exploratory activity. APPs695 and APPs751 were equally effective in the object recognition task, suggesting that the memory-enhancing effect of APPs does not require the Kunitz protease inhibitor domain. These data suggest an important role for APPss on memory processes.
Figures
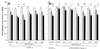


Comment in
-
A role for the beta-amyloid precursor protein in memory?Proc Natl Acad Sci U S A. 1998 Oct 13;95(21):12074-6. doi: 10.1073/pnas.95.21.12074. Proc Natl Acad Sci U S A. 1998. PMID: 9770440 Free PMC article. No abstract available.
References
-
- Selkoe D J. Annu Rev Cell Biol. 1994;10:373–403. - PubMed
-
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindelhurst C, et al. Nature (London) 1992;359:325–327. - PubMed
-
- Li H L, Roch J M, Sundsmo M, Otero D, Sisodia S, Thomas R, Saitoh T. J Neurobiol. 1997;32:469–480. - PubMed
-
- Smith-Swintosky V, Pettigrew C, Craddock S D, Culwell A R, Rydel R E, Mattson M P. J Neurochem. 1994;63:781–784. - PubMed
-
- Goodman Y, Mattson M P. Exp Neurol. 1994;128:1–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

