Direct detection of respiratory syncytial virus, parainfluenza virus, and adenovirus in clinical respiratory specimens by a multiplex reverse transcription-PCR assay
- PMID: 9774555
- PMCID: PMC105291
- DOI: 10.1128/JCM.36.11.3149-3154.1998
Direct detection of respiratory syncytial virus, parainfluenza virus, and adenovirus in clinical respiratory specimens by a multiplex reverse transcription-PCR assay
Abstract
Diagnosis of respiratory virus infections currently involves detection by isolation or antigen detection, which usually identifies only a single suspected agent. To permit identification of more than one respiratory virus in clinical specimens, a rapid detection method involving a single-step, multiplex reverse transcription-PCR (RT-PCR) assay was developed. The assay included five primer sets that amplified the RNA of respiratory syncytial virus subtypes A and B, parainfluenza virus types 1, 2, and 3, and adenovirus types 1 to 7. Initially the assay was tested on tissue culture-grown virus and was found to be specific for all 12 prototype viruses tested, with no interassay cross amplification or amplification of other respiratory viruses. Assay sensitivity allowed a detection range of 0.2 50% tissue culture infectious dose (TCID50) for adenovirus to 250 TCID50 for parainfluenza virus type 1. The multiplex RT-PCR assay was also able to directly detect viruses in respiratory specimens, with virus being detected in 41 of 112 samples as compared to 34 of 112 samples detected by direct immunofluorescence or antigen detection following specimen culture. This suggests that the multiplex RT-PCR assay can be used as a rapid and sensitive diagnostic method for major respiratory viruses.
Figures
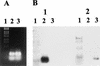
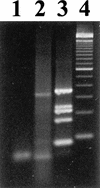

References
-
- Adcock P M, Stout G G, Hauck M A, Marshall G S. Effect of rapid viral diagnosis on the management of children hospitalized with lower respiratory tract infection. Pediatr Infect Dis J. 1997;16:842–846. - PubMed
-
- Belshe R B, Newman F K, Ray R. Parainfluenza virus vaccines. In: Kiyano H, Ogra P L, McGhee J R, editors. Mucosal vaccines. London, United Kingdom: Academic Press, Inc.; 1996. pp. 311–323.
-
- Dowell S F, Anderson L J, Gary H E, Jr, Erdman D D, Plouffe J F, File T M, Jr, Marston B J, Breiman R F. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174:456–462. - PubMed
-
- Eugene-Ruellan G, Freymuth F, Bahloul C, Badrane H, Vabret A, Tordo N. Detection of respiratory syncytial virus A and B and parainfluenzavirus 3 sequences in respiratory tracts of infants by a single PCR with primers targeted to the l-polymerase gene and differential hybridization. J Clin Microbiol. 1998;36:796–801. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

