Comparison of the MB/BacT system with a revised antibiotic supplement kit to the BACTEC 460 system for detection of mycobacteria in clinical specimens
- PMID: 9774571
- PMCID: PMC105307
- DOI: 10.1128/JCM.36.11.3234-3238.1998
Comparison of the MB/BacT system with a revised antibiotic supplement kit to the BACTEC 460 system for detection of mycobacteria in clinical specimens
Abstract
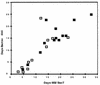
The MB/BacT system (MB/BacT) with a revised antibiotic supplement kit was compared with the BACTEC 460 system (BACTEC 460) in a test of 488 specimens submitted for mycobacterial culture from 302 patients. Twenty-four Mycobacterium tuberculosis isolates were detected by the BACTEC 460 versus 23 isolates by the MB/BacT. Mean time until detection of M. tuberculosis isolates identified by both systems was 11.9 days for the BACTEC 460 versus 13.7 days for the MB/BacT (P = 0.046). M. avium complex was detected in 12 specimens by the MB/BacT versus 10 specimens by the BACTEC 460. Only 8 of 14 (57%) M. avium isolates were detected by both systems, with a mean time until detection of 10.1 days for the BACTEC 460 and 14.2 days for the MB/BacT (P = 0.009). The BACTEC 460 and the MB/BacT detected M. gordonae in four specimens, but only a single specimen was positive by both systems. One M. fortuitum isolate and one of five M. kansasii isolates were recovered only by the BACTEC 460. The bacterial overgrowth rate was 7.0% for the MB/BacT versus 4.1% for the BACTEC 460. We found the MB/BacT to be comparable to the BACTEC 460 for mycobacterial detection. Even though time until detection with the MB/BacT was slightly longer (1.8 days longer for M. tuberculosis and 4.1 days for M. avium [mean values]) and the bacterial overgrowth rate was somewhat higher, the decreased labor, the availability of a computerized data management system, and the noninvasive, nonradiometric aspects of the MB/BacT offset these relative disadvantages and make it an acceptable alternative for use in the diagnostic laboratory.
Figures
References
-
- Centers for Disease Control and Prevention. Provisional cases of selected notifiable diseases, United States, weeks ending Dec 27, 1997 and Dec 28, 1996 (52nd week) Morbid Mortal Weekly Rep. 1997;46:1261.
-
- Centers for Disease Control and Prevention-National Institutes of Health. Biosafety in microbiological and biomedical laboratories. 3rd ed. U.S. Department of Health and Human Services publication no. (CDC) 93-8395 1993. , Atlanta, Ga.
-
- Couchot K, Talbot R. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Direct DNA probe of MB/BacT mycobacterial culture system bottles for Mycobacterium avium complex and Mycobacterium tuberculosis complex, abstr. U-7; p. 102.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

