Comparison of PCR- and hybrid capture-based human papillomavirus detection systems using multiple cervical specimen collection strategies
- PMID: 9774574
- PMCID: PMC105310
- DOI: 10.1128/JCM.36.11.3248-3254.1998
Comparison of PCR- and hybrid capture-based human papillomavirus detection systems using multiple cervical specimen collection strategies
Erratum in
- J Clin Microbiol 1999 Feb;37(2):478
Abstract
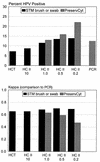
This study compared the performances of three human papillomavirus (HPV) detection tests with specimens collected by three alternative procedures. The HPV tests included the Hybrid Capture Tube test (HCT), the microplate-based Hybrid Capture II test (HC II), and the MY09-MY11 L1 consensus primer PCR-based assay. Initial cervical specimens were collected from study subjects with a broom device, and after Papanicolaou smears were made, residual specimens were placed into PreservCyt (PC), a liquid cytology medium. A second specimen was collected from each subject and placed into Digene Specimen Transport Medium (STM). The device for collection of the second specimen alternated with consecutive subjects between a conical cytology brush and a Dacron swab. At the 1.0-pg/ml cutoff, the results of the HC II agreed well with those of the PCR. Specifically, when PCR data were restricted to the types found by the HC II (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), there was greater than 90% agreement between the HC II and PCR results with both STM and PC. At a lower cutoff (0.2 pg/ml), HC II-positive results increased further, especially when the test was applied to the PC specimens. However, false-positive HC II results were more often observed at the 0.2-pg/ml cutoff. HC II yielded the highest HPV positivity with specimens placed into PC, followed by specimens collected with a conical brush and placed into STM and, last, by those collected with a Dacron swab and placed into STM. Our results demonstrate the utility of both the STM and PC specimen collection methods and show good agreement between the HC II and PCR.
Figures
References
-
- Bauer H M, Manos M M. PCR detection of genital human papillomavirus. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology. Washington, D.C: ASM Press; 1993. pp. 407–413.
-
- Bauer H M, Ting Y, Greer C E, Chambers J C, Tashiro C J, Chimera J, Reingold A, Manos M M. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991;265:472–476. - PubMed
-
- Bernard H U, Chan S Y, Manos M M, Ong C K, Villa L L, Delius H, Peyton C L, Bauer H M, Wheeler C M. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J Infect Dis. 1994;170:1077–1085. - PubMed
-
- Bosch F, Manos M M, Muñoz N, Sherman M, Jansen A M, Peto J, Schiffman M H, Moreno V, Kurman R, Shah K V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995;87:796–802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

