Retinoic acid receptor gamma1 (RARgamma1) levels control RARbeta2 expression in SK-N-BE2(c) neuroblastoma cells and regulate a differentiation-apoptosis switch
- PMID: 9774664
- PMCID: PMC109234
- DOI: 10.1128/MCB.18.11.6482
Retinoic acid receptor gamma1 (RARgamma1) levels control RARbeta2 expression in SK-N-BE2(c) neuroblastoma cells and regulate a differentiation-apoptosis switch
Abstract
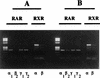
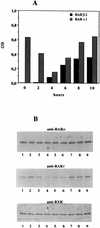
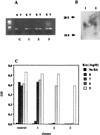
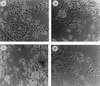
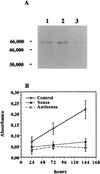
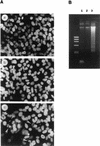
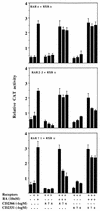
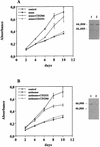
Vitamin A and its derivatives (retinoids) have profound effects on the proliferation and differentiation of many cell types and are involved in a diverse array of developmental and physiological regulatory processes, including those responsible for the development of the mature nervous system. Retinoid signals are mediated by retinoic acid (RA) receptors (RARs) and retinoid X receptors (RXRs), which show distinct spatio-temporal patterns of expression during development and in adult tissues. We have used SK-N-BE2(c) neuroblastoma cells to study the effects of reciprocal regulation of expression of various RARs. We show that in these cells RARgamma1 acts as a repressor of RARbeta2 transcription in the absence of an agonist. In the presence of RA, the expression of RARgamma1 is reduced and that of RARbeta2 is induced. Overexpression of RARgamma1 neutralizes the effects of RA on RARbeta induction. Expression of an RARgamma1-specific antisense construct leads to the constitutive expression of RARbeta2. Although both overexpression of RARgamma1 and its reduction of expression can result in inhibition of cell proliferation, they induce different morphological changes. Reduction of RARgamma1 (and induction of RARbeta) leads to increased apoptosis, whereas RARgamma1 overexpression leads to differentiation in the absence of apoptosis. Thus, RARgamma1 appears to control a differentiation-apoptosis switch in SK-N-BE2(c) neuroblastoma cells.
Figures

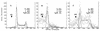
References
-
- Abemayor E, Chang B, Sidell N. Effects of retinoic acid on the in vivo growth of human neuroblastoma cells. Cancer Lett. 1990;55:1–5. - PubMed
-
- Alles A J, Sulik K K. Retinoic acid-induced spina bifida: evidence for a pathogenetic mechanism. Development. 1990;108:73–81. - PubMed
-
- Biedler J L, Helson L, Spengler B A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973;33:2643–2652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
