Poly(A) tail length control in Saccharomyces cerevisiae occurs by message-specific deadenylation
- PMID: 9774670
- PMCID: PMC109240
- DOI: 10.1128/MCB.18.11.6548
Poly(A) tail length control in Saccharomyces cerevisiae occurs by message-specific deadenylation
Abstract
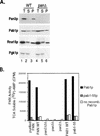
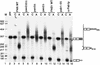
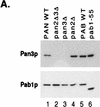
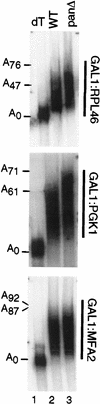
We report that newly synthesized mRNA poly(A) tails are matured to precise lengths by the Pab1p-dependent poly(A) nuclease (PAN) of Saccharomyces cerevisiae. These results provide evidence for an initial phase of mRNA deadenylation that is required for poly(A) tail length control. In RNA 3'-end processing extracts lacking PAN, transcripts are polyadenylated to lengths exceeding 200 nucleotides. By contrast, in extracts containing PAN, transcripts were produced with the expected wild-type poly(A) tail lengths of 60 to 80 nucleotides. The role for PAN in poly(A) tail length control in vivo was confirmed by the finding that mRNAs are produced with longer poly(A) tails in PAN-deficient yeast strains. Interestingly, wild-type yeast strains were found to produce transcripts which varied in their maximal poly(A) tail length, and this message-specific length control was lost in PAN-deficient strains. Our data support a model whereby mRNAs are polyadenylated by the 3'-end processing machinery with a long tail, possibly of default length, and then in a PAN-dependent manner, the poly(A) tails are rapidly matured to a message-specific length. The ability to control the length of the poly(A) tail for newly expressed mRNAs has the potential to be an important posttranscriptional regulatory step in gene expression.
Figures





References
-
- Astrom J, Astrom A, Virtanen A. Properties of a HeLa cell 3′ exonuclease specific for degrading poly(A) tails of mammalian mRNA. J Biol Chem. 1992;267:18154–18159. - PubMed
-
- Baker E J. Control of poly(A) length. In: Belasco J G, Brawerman G, editors. Control of messenger RNA stability. San Diego, Calif: Academic Press, Inc.; 1993. pp. 367–415.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
