Physical and functional interactions between type I transforming growth factor beta receptors and Balpha, a WD-40 repeat subunit of phosphatase 2A
- PMID: 9774674
- PMCID: PMC109244
- DOI: 10.1128/MCB.18.11.6595
Physical and functional interactions between type I transforming growth factor beta receptors and Balpha, a WD-40 repeat subunit of phosphatase 2A
Abstract
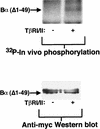
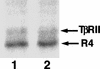
We have previously shown that a WD-40 repeat protein, TRIP-1, associates with the type II transforming growth factor beta (TGF-beta) receptor. In this report, we show that another WD-40 repeat protein, the Balpha subunit of protein phosphatase 2A, associates with the cytoplasmic domain of type I TGF-beta receptors. This association depends on the kinase activity of the type I receptor, is increased by coexpression of the type II receptor, which is known to phosphorylate and activate the type I receptor, and allows the type I receptor to phosphorylate Balpha. Furthermore, Balpha enhances the growth inhibition activity of TGF-beta in a receptor-dependent manner. Because Balpha has been characterized as a regulator of phosphatase 2A activity, our observations suggest possible functional interactions between the TGF-beta receptor complex and the regulation of protein phosphatase 2A.
Figures





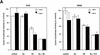
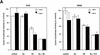
References
-
- Alessi D R, Gomez N, Moorhead G, Lewis T, Keyse S M, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol. 1995;5:283–295. - PubMed
-
- Andersson S, Davis D L, Dahlback H, Jornvall H, Russell D W. Cloning, structure, and expression of the mitrochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. - PubMed
-
- Bassing C H, Yingling J M, Howe D J, Wang T, He W W, Gustafson M L, Shah P, Donahoe P K, Wang X-F. A transforming growth factor β type I receptor that signals to activate gene expression. Science. 1993;263:87–89. - PubMed
-
- Brand T, Schneidert M D. Inactive type II and type I receptors for TGF β are dominant inhibitors of TGF beta-dependent transcription. J Biol Chem. 1995;270:8274–8284. - PubMed
-
- Cairns J, Qin S, Philp R, Tan Y H, Guy G R. Dephosphorylation of the small heat shock protein Hsp27 in vivo by protein phosphatase 2A. J Biol Chem. 1994;269:9176–9183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
