Angiogenesis in mice with chronic airway inflammation: strain-dependent differences
- PMID: 9777941
- PMCID: PMC1853051
- DOI: 10.1016/S0002-9440(10)65654-4
Angiogenesis in mice with chronic airway inflammation: strain-dependent differences
Abstract
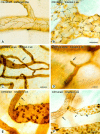
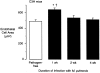
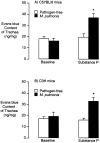
Chronic inflammation is associated with blood vessel proliferation and enlargement and changes in vessel phenotype. We sought to determine whether these changes represent different types of angiogenesis and whether they are stimulus dependent. Chronic airway inflammation, produced by infection with Mycoplasma pulmonis, was compared in strains of mice known to be resistant (C57BL/6) or susceptible (C3H). Tracheal vascularity, assessed in whole mounts after Lycopersicon esculentum lectin staining, increased in both strains at 1, 2, 4, and 8 weeks after infection, but the type of vascular remodeling was different. The number of vessels doubled in tracheas of C57BL/6 mice, with corresponding increases of capillaries and venules. In contrast, neither the number nor the length of vessels changed in C3H mice. Instead, vessel diameter and endothelial cell number doubled, and the proportion of venules doubled with a corresponding decrease of capillaries. Although the infection had no effect on baseline plasma leakage, in both strains it potentiated the leakage produced by substance P. We conclude that the same stimulus can result in blood vessel proliferation or enlargement, depending on the host response. Endothelial cells proliferate in both cases, but in one case new capillaries form whereas in the other capillaries convert to venules.
Figures






Comment in
-
Chronic inflammation: links with angiogenesis and wound healing.Am J Pathol. 1998 Oct;153(4):1035-9. doi: 10.1016/S0002-9440(10)65648-9. Am J Pathol. 1998. PMID: 9777935 Free PMC article. Review. No abstract available.
References
-
- Risau W: Mechanisms of angiogenesis. Nature 1997, 386:671-674 - PubMed
-
- Folkman J, Brem H: Angiogenesis and inflammation. ed 2 Gallin JI Goldstein IM Snyderman R eds. Inflammation: Basic Principles and Clinical Correlates, 1992, :pp 821-839 Raven Press, New York
-
- Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD: The co-dependence of angiogenesis and chronic inflammation. FASEB J 1997, 11:457-465 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

