Characterization of monoclonal antibodies to calpain 3 and protein expression in muscle from patients with limb-girdle muscular dystrophy type 2A
- PMID: 9777948
- PMCID: PMC1853046
- DOI: 10.1016/S0002-9440(10)65661-1
Characterization of monoclonal antibodies to calpain 3 and protein expression in muscle from patients with limb-girdle muscular dystrophy type 2A
Abstract
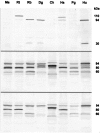
Monoclonal antibodies were raised to two regions of calpain 3 (muscle-specific calcium-activated neutral protease), which is the product of the gene that is defective in limb-girdle muscular dystrophy type 2A. The antibodies produced characteristic patterns of bands on Western blots: normal calpain 3 protein was represented by bands at 94 kd, plus additional fragments at approximately 60 or 30 kd, according to the antibody used. Specificity was confirmed by the loss of all bands in patients with null gene mutations. The "normal" profile of bands was observed in muscle from 33 control subjects and 70 disease-control patients. Calpain 3 protein was found to be extremely stable in fresh human muscle, with full-size protein being detected 8 hours after the muscle had been removed. Blots of muscle from nine limb-girdle muscular dystrophy type 2A patients with defined mutations showed variation in protein expression, with seven showing a clear reduction in the abundance of protein detected. No simple relationship was found between the abundance and clinical severity. Two patients showed normal expression of the full-size 94 kd band accompanied by a clear reduction in the smaller fragments. This pattern was also observed in one patient with an undefined form of limb-girdle dystrophy. These results indicate that immunodiagnosis is feasible, but caution will need to be exercised with the interpretation of near-normal protein profiles.
Figures



References
-
- Noguchi S, McNally EM, Othmane KB, Hagiwara Y, Mizuno Y, Yoshida M, Yamanmoto H, Bönnemann CG, Gussoni E, Denton PH, Kyriakides T, Middleton L, Hentati F, Hamida MB, Nonaka I, Vance JM, Kunkel LM, Ozawa E: Mutations in the dystrophin-associated protein-γ-sarcoglycan in chromosome 13 muscular dystrophy. Science 1995, 270:819-822 - PubMed
-
- Roberds SL, Leturcq F, Allamand V, Piccolo F, Jeanpierre M, Anderson RD, Lim LE, Lee JC, Tomé FMS, Romero NB, Fardeau M, Beckmann JS, Kaplan J-C, Campbell KP: Missense mutations in the adhalin gene linked to autosomal recessive muscular dystrophy. Cell 1994, 78:625-633 - PubMed
-
- Lim LE, Duclos F, Broux O, Bourg N, Sanada Y, Allamand V, Meyer J, Richard I, Moomaw C, Slaughter C, Tomé FMS, Fardeau M, Jackson CE, Beckmann JS, Campbell KP: β-Sarcoglycan: characterization and role in limb-girdle muscular dystrophy linked to 4q12. Nat Genet 1995, 11:257-265 - PubMed
-
- Nigro V, de Sá Moreira E, Piluso G, Vainzof M, Belsito A, Politano L, Puca AA, Passos-Bueno MR, Zatz M: Autosomal recessive limb-girdle muscular dystrophy, LGMD2F, is caused by a mutation in the δ-sarcoglycan gene. Nat Genet 1996, 14:195-198 - PubMed
-
- Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, Brenguier L, Devaud C, Pasturaud P, Roudaut C, Hillaire D, Passos-Bueno MR, Zatz M, Tischfield JA, Fardeau M, Jackson CE, Cohen D, Beckmann JS: Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 1995, 81:27-40 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

