Bikunin present in human peritoneal fluid is in part derived from the interaction of serum with peritoneal mesothelial cells
- PMID: 9777958
- PMCID: PMC1853064
- DOI: 10.1016/S0002-9440(10)65671-4
Bikunin present in human peritoneal fluid is in part derived from the interaction of serum with peritoneal mesothelial cells
Abstract
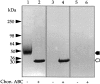
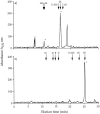
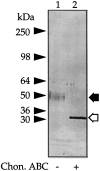
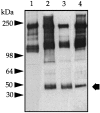
We recently reported that peritoneal fluid mainly contains two proteoglycans; one is the interstitial proteoglycan referred to as decorin, and the other an uncharacterized small chondroitin sulfate proteoglycan. In the present study, we have used a two-step process to isolate the small chondroitin sulfate proteoglycan free of decorin. The purified molecule ran as a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis with apparent molecular mass 50 kd made up of a chondroitin-4-sulfate glycosaminoglycan chain and a 30-kd core protein. NH2-terminal analysis of the core protein showed significant sequence homology with bikunin, a component of the human inter-alpha-trypsin inhibitor (IalphaI) family. A Western blot analysis using anti-human inter-alpha-trypsin inhibitor confirmed the identity of the small chondroitin sulfate proteoglycan as bikunin, and a trypsin inhibitor counterstain assay confirmed its anti-trypsin activity. Examination of serum from patients receiving continuous peritoneal dialysis suggests that free bikunin in peritoneal fluid may be the result of leakage of serum proteins into the peritoneum. Our findings further show that the interaction of serum with peritoneal mesothelial cells offers a new and novel explanation for the presence of bikunin in peritoneal fluid.
Figures






References
-
- Dobbie JW: New concepts in molecular biology and ultrastructural pathology of the peritoneum: their significance for peritoneal dialysis. Am J Kidney Dis 1990, 15:97-109 - PubMed
-
- Dobbie JW, Pavlina T, Lloyd J, Johnson RC: Phosphatidylcholine synthesis by peritoneal mesothelium: its implications for peritoneal dialysis. Am J Kidney Dis 1988, 12:31-36 - PubMed
-
- Beavis J, Harwood J, Coles G, Williams J: Synthesis of phospholipids by human peritoneal mesothelial cells. Perit Dial Int 1994, 14:348-355 - PubMed
-
- Mizusawa Y, Thomas K, Hills B, Burke J, Mizushima W, Rigby R, Crawford C, Freeman J: Proteolipid in peritoneal effluent of CAPD patients. Perit Dial Int 1998, 18:225-228 - PubMed
-
- Douvdevani A, Rapoport J, Konforty A, Argov S, Ovnat A, Chaimovitz C: Human peritoneal mesothelial cells synthesize IL-1α and IL-1β. Kidney Int 1994, 46:993-1001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

