Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow
- PMID: 9782118
- PMCID: PMC2213407
- DOI: 10.1084/jem.188.8.1413
Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow
Abstract
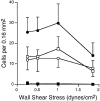
Leukocyte migration into sites of inflammation involves multiple molecular interactions between leukocytes and vascular endothelial cells, mediating sequential leukocyte capture, rolling, and firm adhesion. In this study, we tested the role of molecular interactions between fractalkine (FKN), a transmembrane mucin-chemokine hybrid molecule expressed on activated endothelium, and its receptor (CX3CR1) in leukocyte capture, firm adhesion, and activation under physiologic flow conditions. Immobilized FKN fusion proteins captured resting peripheral blood mononuclear cells at physiologic wall shear stresses and induced firm adhesion of resting monocytes, resting and interleukin (IL)-2-activated CD8(+) T lymphocytes and IL-2-activated NK cells. FKN also induced cell shape change in firmly adherent monocytes and IL-2-activated lymphocytes. CX3CR1-transfected K562 cells, but not control K562 cells, firmly adhered to FKN-expressing ECV-304 cells (ECV-FKN) and tumor necrosis factor alpha-activated human umbilical vein endothelial cells. This firm adhesion was not inhibited by pertussis toxin, EDTA/EGTA, or antiintegrin antibodies, indicating that the firm adhesion was integrin independent. In summary, FKN mediated the rapid capture, integrin-independent firm adhesion, and activation of circulating leukocytes under flow. Thus, FKN and CX3CR1 mediate a novel pathway for leukocyte trafficking.
Figures





References
-
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration, the multistep paradigm. Cell. 1994;76:301–314. - PubMed
-
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. - PubMed
-
- Ley K, Tedder TF. Leukocyte interactions with vascular endothelium: new insights into selectin mediated attachment and rolling. J Immunol. 1995;155:525–528. - PubMed
-
- Tedder TF, Steeber DA, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB (Fed Am Soc Exp Biol) J. 1995;9:866–873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

