Neutrophil-derived 5'-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation
- PMID: 9782120
- PMCID: PMC2213403
- DOI: 10.1084/jem.188.8.1433
Neutrophil-derived 5'-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation
Abstract
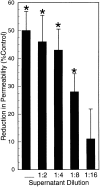
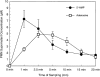
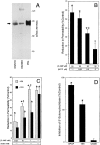
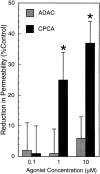
During episodes of inflammation, polymorphonuclear leukocyte (PMN) transendothelial migration has the potential to disturb vascular barrier function and give rise to intravascular fluid extravasation and edema. However, little is known regarding innate mechanisms that dampen fluid loss during PMN-endothelial interactions. Using an in vitro endothelial paracellular permeability model, we observed a PMN-mediated decrease in endothelial paracellular permeability. A similar decrease was elicited by cell-free supernatants from activated PMN (FMLP 10(-6) M), suggesting the presence of a PMN-derived soluble mediator(s). Biophysical and biochemical analysis of PMN supernatants revealed a role for PMN-derived 5'-adenosine monophosphate (AMP) and its metabolite, adenosine, in modulation of endothelial paracellular permeability. Supernatants from activated PMN contained micromolar concentrations of bioactive 5'-AMP and adenosine. Furthermore, exposure of endothelial monolayers to authentic 5'-AMP and adenosine increased endothelial barrier function more than twofold in both human umbilical vein endothelial cells and human microvascular endothelial cells. 5'-AMP bioactivity required endothelial CD73-mediated conversion of 5'-AMP to adenosine via its 5'-ectonucleotidase activity. Decreased endothelial paracellular permeability occurred through adenosine A2B receptor activation and was accompanied by a parallel increase in intracellular cAMP. We conclude that activated PMN release soluble mediators, such as 5'-AMP and adenosine, that promote endothelial barrier function. During inflammation, this pathway may limit potentially deleterious increases in endothelial paracellular permeability and could serve as a basic mechanism of endothelial resealing during PMN transendothelial migration.
Figures






References
-
- Hansen PR. Role of neutrophils in myocardial ischemia and reperfusion. Circulation. 1995;91:1872–1885. - PubMed
-
- Sunnergran KP, Rovetto MJ. Myocyte and endothelial injury with ischemia reperfusion in isolated rat hearts. Am J Physiol. 1987;252:H1211–H1217. - PubMed
-
- Dejana E, Corada M, Lampugnani MG. Endothelial cell-to-cell junctions. FASEB J. 1995;9:910–918. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

