Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells
- PMID: 9782124
- PMCID: PMC2213410
- DOI: 10.1084/jem.188.8.1473
Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells
Abstract
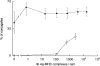
Dendritic cells (DCs) are much more potent antigen (Ag)-presenting cells than resting B cells for the activation of naive T cells. The mechanisms underlying this difference have been analyzed under conditions where ex vivo DCs or B cells presented known numbers of specific Ag-major histocompatibility complex (MHC) complexes to naive CD4(+) T cells from T cell antigen receptor (TCR) transgenic mice. Several hundred Ag-MHC complexes presented by B cells were necessary to elicit the formation of a few T-B conjugates with small contact zones, and the resulting individual T cell Ca2+ responses were all-or-none. In contrast, Ag-specific T cell Ca2+ responses can be triggered by DCs bearing an average of 30 Ag-MHC complexes per cell. Formation of T-DC conjugates is Ag-independent, but in the presence of the Ag, the surface of the contact zone increases and so does the amplitude of the T cell Ca2+ responses. These results suggest that Ag is better recognized by T cells on DCs essentially because T-DC adhesion precedes Ag recognition, whereas T-B adhesion requires Ag recognition. Surprisingly, we also recorded small Ca2+ responses in T cells interacting with unpulsed DCs. Using DCs purified from MHC class II knockout mice, we provide evidence that this signal is mostly due to MHC-TCR interactions. Such an Ag-independent, MHC-triggered calcium response could be a survival signal that DCs but not B cells are able to deliver to naive T cells.
Figures








References
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

