CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells
- PMID: 9782130
- PMCID: PMC2213414
- DOI: 10.1084/jem.188.8.1529
CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells
Abstract
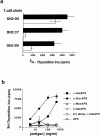
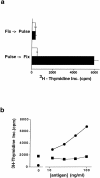
A conserved subset of mature circulating T cells in humans expresses an invariant Valpha24-JalphaQ T cell receptor (TCR)-alpha chain rearrangement and several natural killer (NK) locus-encoded C-type lectins. These human T cells appear to be precise homologues of the subset of NK1.1(+) TCR-alpha/beta+ T cells, often referred to as NK T cells, which was initially identified in mice. Here we show that human NK T cell clones are strongly and specifically activated by the same synthetic glycolipid antigens as have been shown recently to stimulate murine NK T cells. Responses of human NK T cells to these synthetic glycolipids, consisting of certain alpha-anomeric sugars conjugated to an acylated phytosphingosine base, required presentation by antigen-presenting cells expressing the major histocompatibility complex class I-like CD1d protein. Presentation of synthetic glycolipid antigens to human NK T cells required internalization of the glycolipids by the antigen-presenting cell and normal endosomal targeting of CD1d. Recognition of these compounds by human NK T cells triggered proliferation, cytokine release, and cytotoxic activity. These results demonstrate a striking parallel in the specificity of NK T cells in humans and mice, thus providing further insight into the potential mechanisms of immune recognition by NK T cells and the immunological function of this unique T cell subset.
Figures


References
-
- Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. - PubMed
-
- Prussin C, Foster B. TCR Vα24 and Vβ11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J Immunol. 1997;159:5862–5870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

