An intercellular regenerative calcium wave in porcine coronary artery endothelial cells in primary culture
- PMID: 9782162
- PMCID: PMC2231269
- DOI: 10.1111/j.1469-7793.1998.103by.x
An intercellular regenerative calcium wave in porcine coronary artery endothelial cells in primary culture
Abstract
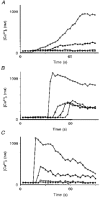
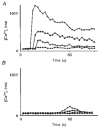
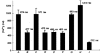
1. A regenerative calcium wave is an increase in cytosolic free calcium concentration ([Ca2+]i) which extends beyond the stimulated cells without decrement of amplitude, kinetics of [Ca2+]i increase and speed of propagation. 2. The aim of the present study was to test the hypothesis that such a wave could be evoked by bradykinin stimulation and by scraping cultured endothelial cells from porcine coronary arteries. 3. Calcium imaging was performed using the calcium-sensitive dye fura-2. A wound or a delivery of bradykinin to two to three cells on growing clusters of approximately 300 cells caused an increase in [Ca2+]i which was propagated throughout the cluster in a regenerative manner over distances up to 400 micrometer. This wave spread through gap junctions since it was inhibited by the cell uncoupler palmitoleic acid. 4. The same experiments performed in confluent cultures caused a rise in [Ca2+]i which failed to propagate in a regenerative way. The wave propagation probably failed because the confluent cells were less dye coupled than the growing cells. This was confirmed by immunohistology which detected a dramatic decrease in the number of connexin 40 gap junctions in the confluent cultures. 5. The regenerative propagation of the wave was blocked by inhibitors of calcium-induced calcium release (CICR) and phospholipase C (PLC), and by suppression of extracellular calcium, but not by clamping the membrane potential with high-potassium solution. 6. We conclude that regenerative intercellular calcium waves exist in cultured islets but not in confluent cultures of endothelial cells. An increase in [Ca2+]i is not sufficient to trigger a regenerative propagation. The PLC pathway, CICR and extracellular calcium are all necessary for a fully regenerated propagation.
Figures
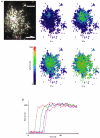
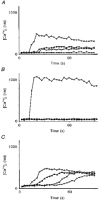

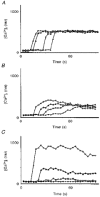
Similar articles
-
Ca(2+)-dependent non-selective cation and potassium channels activated by bradykinin in pig coronary artery endothelial cells.J Physiol. 1996 Jun 15;493 ( Pt 3)(Pt 3):691-706. doi: 10.1113/jphysiol.1996.sp021415. J Physiol. 1996. PMID: 8799892 Free PMC article.
-
Substance P and bradykinin activate different types of KCa currents to hyperpolarize cultured porcine coronary artery endothelial cells.J Physiol. 1999 Sep 1;519 Pt 2(Pt 2):361-71. doi: 10.1111/j.1469-7793.1999.0361m.x. J Physiol. 1999. PMID: 10457055 Free PMC article.
-
Membrane potential and Na(+)-K+ pump activity modulate resting and bradykinin-stimulated changes in cytosolic free calcium in cultured endothelial cells from bovine atria.J Biol Chem. 1990 Feb 15;265(5):2613-9. J Biol Chem. 1990. PMID: 2154452
-
Intercellular Ca(2+) wave propagation in human retinal pigment epithelium cells induced by mechanical stimulation.Exp Eye Res. 2013 Mar;108:129-39. doi: 10.1016/j.exer.2013.01.009. Epub 2013 Jan 22. Exp Eye Res. 2013. PMID: 23352832
-
[Intra- and intercellular Ca(2+)-signal transduction].Verh K Acad Geneeskd Belg. 2000;62(6):501-63. Verh K Acad Geneeskd Belg. 2000. PMID: 11196579 Review. Dutch.
Cited by
-
Intercellular Ca(2+) waves: mechanisms and function.Physiol Rev. 2012 Jul;92(3):1359-92. doi: 10.1152/physrev.00029.2011. Physiol Rev. 2012. PMID: 22811430 Free PMC article. Review.
-
Intercellular ultrafast Ca(2+) wave in vascular smooth muscle cells: numerical and experimental study.Sci Rep. 2016 Aug 10;6:31271. doi: 10.1038/srep31271. Sci Rep. 2016. PMID: 27507785 Free PMC article.
-
P-Selectin and ICAM-1 synergy in mediating THP-1 monocyte adhesion in hemodynamic flow is length dependent.Integr Biol (Camb). 2017 Apr 18;9(4):313-327. doi: 10.1039/c7ib00020k. Integr Biol (Camb). 2017. PMID: 28262902 Free PMC article.
-
An evaluation of potassium ions as endothelium-derived hyperpolarizing factor in porcine coronary arteries.Br J Pharmacol. 2000 Nov;131(5):965-73. doi: 10.1038/sj.bjp.0703658. Br J Pharmacol. 2000. PMID: 11053218 Free PMC article.
-
Smooth muscle mediates circumferential conduction of hyperpolarization and relaxation to focal endothelial cell activation in large coronary arteries.Naunyn Schmiedebergs Arch Pharmacol. 2007 Apr;375(2):85-94. doi: 10.1007/s00210-007-0149-7. Epub 2007 Mar 6. Naunyn Schmiedebergs Arch Pharmacol. 2007. PMID: 17340126
References
-
- Allbritton NL, Meyer T. Localized calcium spikes and propagating calcium waves. Cell Calcium. 1993;14:691–697. 10.1016/0143-4160(93)90095-N. - DOI - PubMed
-
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. - PubMed
-
- Bény J-L, Pacicca C. Bi-directional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. American Journal of Physiology. 1994;266:H1465–1472. - PubMed
-
- Berridge MJ. Calcium signaling and cell proliferation. BioEssays. 1995;17:491–500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

