Role of cAMP-dependent protein kinase A in activation of a voltage-sensitive release mechanism for cardiac contraction in guinea-pig myocytes
- PMID: 9782169
- PMCID: PMC2231262
- DOI: 10.1111/j.1469-7793.1998.185by.x
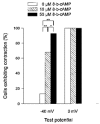
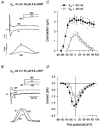
Role of cAMP-dependent protein kinase A in activation of a voltage-sensitive release mechanism for cardiac contraction in guinea-pig myocytes
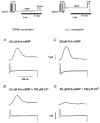
Abstract
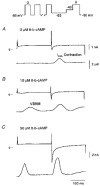
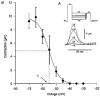
1. Ionic currents and unloaded cell shortening were recorded from guinea-pig ventricular myocytes with single electrode voltage clamp techniques and video edge detection at 37 C. Patch pipettes (1-3 MOmega) were used to provide intracellular dialysis with pipette solutions. 2. Na+ currents were blocked with 200 microM lidocaine. Contractions initiated by the voltage-sensitive release mechanism (VSRM) and Ca2+-induced Ca2+ release (CICR) in response to L-type Ca2+ current (ICa,L) were separated with voltage clamp protocols. 3. Without 8-bromo cyclic adenosine 3',5'-monophosphate (8-Br-cAMP) in the pipette, small VSRM-induced contractions occurred transiently in only 13% of myocytes. In contrast, large ICa,L-induced contractions were demonstrable in 100% of cells. 4. Addition of 10 or 50 microM 8-Br-cAMP to the pipette increased the percentage of cells exhibiting VSRM contractions to 68 and 93%, respectively. With 50 microM 8-Br-cAMP, contractions initiated by the VSRM and ICa,L were not significantly different in amplitude. 5. 8-Br-cAMP-supported VSRM contractions had characteristics of the VSRM shown previously in undialysed myocytes. Cd2+ (100 microM) blocked ICa,L and ICa,L contractions but not VSRM contractions. 8-Br-cAMP-supported contractions exhibited steady-state inactivation with parameters characteristic of the VSRM, as well as sigmoidal contraction-voltage relations. 6. Without 8-Br-cAMP in the pipette, contraction-voltage relations determined with steps from a post-conditioning potential (Vpc) of either -40 or -65 mV were bell shaped, with a threshold near -35 mV. With 50 microM 8-Br-cAMP in the pipette, contraction-voltage relations from a Vpc of -65 mV were sigmoidal and the threshold shifted to near -55 mV. Contraction-voltage relations remained bell shaped in the presence of 8-Br-cAMP when the Vpc was -40 mV. 7. H-89, which inhibits cAMP-dependent protein kinase A (PKA), significantly reduced the amplitudes of VSRM contractions by approximately 84% with 50 microM 8-Br-cAMP in the pipette. H-89 also significantly reduced the amplitudes of peak ICa, L and ICa,L contractions, although to a lesser extent. 8. We conclude that intracellular dialysis with patch pipettes disrupts the adenylyl cyclase-PKA phosphorylation cascade, and that the VSRM requires intracellular phosphorylation to be available for activation. Intracellular dialysis with solutions that do not maintain phosphorylation levels inhibits a major mechanism in cardiac excitation- contraction coupling.
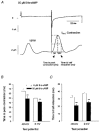
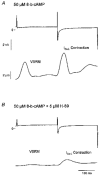
Figures



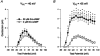

Similar articles
-
Regulation of a voltage-sensitive release mechanism by Ca(2+)-calmodulin-dependent kinase in cardiac myocytes.Am J Physiol Heart Circ Physiol. 2000 Nov;279(5):H2104-15. doi: 10.1152/ajpheart.2000.279.5.H2104. Am J Physiol Heart Circ Physiol. 2000. PMID: 11045943
-
Contractions in guinea-pig ventricular myocytes triggered by a calcium-release mechanism separate from Na+ and L-currents.J Physiol. 1995 Apr 1;484 ( Pt 1)(Pt 1):107-22. doi: 10.1113/jphysiol.1995.sp020651. J Physiol. 1995. PMID: 7602513 Free PMC article.
-
Contribution of a voltage-sensitive calcium release mechanism to contraction in cardiac ventricular myocytes.Am J Physiol. 1998 Jan;274(1):H155-70. doi: 10.1152/ajpheart.1998.274.1.H155. Am J Physiol. 1998. PMID: 9458864
-
Cardiac excitation-contraction coupling: role of membrane potential in regulation of contraction.Am J Physiol Heart Circ Physiol. 2001 May;280(5):H1928-44. doi: 10.1152/ajpheart.2001.280.5.H1928. Am J Physiol Heart Circ Physiol. 2001. PMID: 11299192 Review.
-
Regulation of slow calcium channels of myocardial cells and vascular smooth muscle cells by cyclic nucleotides and phosphorylation.Mol Cell Biochem. 1994 Nov 23;140(2):103-17. doi: 10.1007/BF00926749. Mol Cell Biochem. 1994. PMID: 7898483 Review.
Cited by
-
The voltage-sensitive release mechanism of excitation contraction coupling in rabbit cardiac muscle is explained by calcium-induced calcium release.J Gen Physiol. 2003 May;121(5):353-73. doi: 10.1085/jgp.200208764. J Gen Physiol. 2003. PMID: 12719483 Free PMC article.
-
Propranolol regulates cardiac transient outward potassium channel in rat myocardium via cAMP/PKA after short-term but not after long-term ischemia.Naunyn Schmiedebergs Arch Pharmacol. 2010 Jul;382(1):63-71. doi: 10.1007/s00210-010-0520-y. Epub 2010 May 25. Naunyn Schmiedebergs Arch Pharmacol. 2010. PMID: 20499050
-
Another trigger for the heartbeat.J Physiol. 1998 Nov 15;513 ( Pt 1)(Pt 1):1. doi: 10.1111/j.1469-7793.1998.001by.x. J Physiol. 1998. PMID: 9782153 Free PMC article. No abstract available.
-
Regulation of cardiac excitation and contraction by p21 activated kinase-1.Prog Biophys Mol Biol. 2008 Oct-Nov;98(2-3):238-50. doi: 10.1016/j.pbiomolbio.2009.01.007. Epub 2009 Jan 24. Prog Biophys Mol Biol. 2008. PMID: 19351515 Free PMC article. Review.
-
Tetracaine can inhibit contractions initiated by a voltage-sensitive release mechanism in guinea-pig ventricular myocytes.J Physiol. 1999 Sep 15;519 Pt 3(Pt 3):851-65. doi: 10.1111/j.1469-7793.1999.0851n.x. J Physiol. 1999. PMID: 10457096 Free PMC article.
References
-
- Barcenas-Ruiz L, Wier WG. Voltage-dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circulation Research. 1987;61:148–154. - PubMed
-
- Bassani RA, Mattiazzi A, Bers DM. CaMKII is responsible for activity-dependent acceleration of relaxation in rat ventricular myocytes. American Journal of Physiology. 1995;268:H703–712. - PubMed
-
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

