The maltose-binding protein as a scaffold for monovalent display of peptides derived from phage libraries
- PMID: 9784192
- PMCID: PMC3998728
- DOI: 10.1006/abio.1998.2793
The maltose-binding protein as a scaffold for monovalent display of peptides derived from phage libraries
Erratum in
- Anal Biochem 1999 Jan 15;266(2):240
Abstract
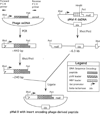
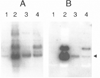
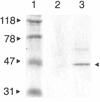
Random peptide libraries are displayed on filamentous bacteriophage as fusions to either the minor coat protein, pIII, or the major coat protein, pVIII. We have devised a means of isolating the peptide displayed on a phage clone by transferring it to the N-terminus of the maltose-binding protein (MBP) of Escherichia coli encoded by malE. Transfer of a peptide sequence to monomeric MBP eliminates phage-encoded amino acids downstream of the insert peptide as well as avidity effects caused by multivalent display on phage. Peptide:MBP fusions are also easily affinity purified on amylose columns. The pMal-p2 vector was engineered to accept phage DNA encoding pIII- and pVIII-displayed peptides fused to their respective leader sequences. Both types of leader sequence were shown to target the peptide:MBP fusions to the periplasm of E. coli. A streamlined procedure for transferring peptides to MBP was applied to clones that had been isolated from a panel of pVIII-displayed peptide libraries by screening with an HIV-1-specific monoclonal antibody (Ab). By enzyme-linked immunosorbent assay, the Ab bound each of the peptide:MBP fusions and required the presence of a disulfide bridge within each peptide. Some of the peptide:MBP fusions were also analyzed using surface plasmon resonance. Thus, our study shows the value of malE fusion vectors in characterizing phage-displayed peptides.
Copyright 1998 Academic Press.
Figures


Similar articles
-
Vectors to facilitate the creation of translational fusions to the maltose-binding protein of Escherichia coli.Gene. 1994 Jun 24;144(1):69-73. doi: 10.1016/0378-1119(94)90205-4. Gene. 1994. PMID: 8026760
-
Homodimeric peptides displayed by the major coat protein of filamentous phage.J Mol Biol. 2000 Jul 7;300(2):307-20. doi: 10.1006/jmbi.2000.3850. J Mol Biol. 2000. PMID: 10873467
-
Selection of a high affinity angiogenin-binding peptide from a peptide library displayed on phage coat protein.Mol Cells. 1997 Oct 31;7(5):575-81. Mol Cells. 1997. PMID: 9387141
-
Production of active chimeric pediocin AcH in Escherichia coli in the absence of processing and secretion genes from the Pediococcus pap operon.Appl Environ Microbiol. 1998 Jan;64(1):14-20. doi: 10.1128/AEM.64.1.14-20.1998. Appl Environ Microbiol. 1998. PMID: 9435056 Free PMC article.
-
Export of the periplasmic maltose-binding protein of Escherichia coli.J Bioenerg Biomembr. 1990 Jun;22(3):401-39. doi: 10.1007/BF00763175. J Bioenerg Biomembr. 1990. PMID: 2202725 Review.
Cited by
-
Random-peptide libraries and antigen-fragment libraries for epitope mapping and the development of vaccines and diagnostics.Curr Opin Chem Biol. 2001 Jun;5(3):314-24. doi: 10.1016/s1367-5931(00)00208-8. Curr Opin Chem Biol. 2001. PMID: 11479124 Free PMC article. Review.
-
A peptide inhibitor of HIV-1 neutralizing antibody 2G12 is not a structural mimic of the natural carbohydrate epitope on gp120.FASEB J. 2008 May;22(5):1380-92. doi: 10.1096/fj.07-8983com. Epub 2008 Jan 15. FASEB J. 2008. PMID: 18198210 Free PMC article.
-
In vivo analysis of an essential archaeal signal recognition particle in its native host.J Bacteriol. 2002 Jun;184(12):3260-7. doi: 10.1128/JB.184.12.3260-3267.2002. J Bacteriol. 2002. PMID: 12029042 Free PMC article.
-
Inhibiting complex IL-17A and IL-17RA interactions with a linear peptide.Sci Rep. 2016 May 17;6:26071. doi: 10.1038/srep26071. Sci Rep. 2016. PMID: 27184415 Free PMC article.
-
The efficiency of Escherichia coli selenocysteine insertion is influenced by the immediate downstream nucleotide.Nucleic Acids Res. 2000 Feb 1;28(3):755-61. doi: 10.1093/nar/28.3.755. Nucleic Acids Res. 2000. PMID: 10637327 Free PMC article.
References
-
- Scott JK, Craig L. Curr. Opin. Biotechnol. 1994;5:40–48. - PubMed
-
- Smith GP, Petrenko VA. Chem. Rev. 1997;97:391–410. - PubMed
-
- Malik P, Terry TD, Gowda LR, Langara A, Petukhov SA, Symmons MF, Welsh LC, Marvin DA, Perham RN. J. Mol. Biol. 1996;260:9–21. - PubMed
-
- Bonnycastle LL, Mehroke JS, Rashed M, Gong X, Scott JK. J. Mol. Biol. 1996;258:747–762. - PubMed
-
- Zhong G, Smith GP, Berry J, Brunham RC. J. Biol. Chem. 1994;269:24183–24188. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

