SXR, a novel steroid and xenobiotic-sensing nuclear receptor
- PMID: 9784494
- PMCID: PMC317212
- DOI: 10.1101/gad.12.20.3195
SXR, a novel steroid and xenobiotic-sensing nuclear receptor
Abstract
An important requirement for physiologic homeostasis is the detoxification and removal of endogenous hormones and xenobiotic compounds with biological activity. Much of the detoxification is performed by cytochrome P-450 enzymes, many of which have broad substrate specificity and are inducible by hundreds of different compounds, including steroids. The ingestion of dietary steroids and lipids induces the same enzymes; therefore, they would appear to be integrated into a coordinated metabolic pathway. Instead of possessing hundreds of receptors, one for each inducing compound, we propose the existence of a few broad specificity, low-affinity sensing receptors that would monitor aggregate levels of inducers to trigger production of metabolizing enzymes. In support of this model, we have isolated a novel nuclear receptor, termed the steroid and xenobiotic receptor (SXR), which activates transcription in response to a diversity of natural and synthetic compounds. SXR forms a heterodimer with RXR that can bind to and induce transcription from response elements present in steroid-inducible cytochrome P-450 genes and is expressed in tissues in which these catabolic enzymes are expressed. These results strongly support the steroid sensor hypothesis and suggest that broad specificity sensing receptors may represent a novel branch of the nuclear receptor superfamily.
Figures



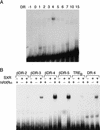
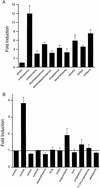

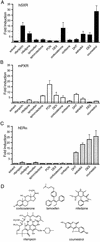

References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Baker ME. Endocrine activity of plant-derived compounds: An evolutionary perspective. Proc Soc Exp Biol Med. 1995;208:131–138. - PubMed
-
- Bammel A, van der Mee K, Ohnhaus EE, Kirch W. Divergent effects of different enzyme-inducing agents on endogenous and exogenous testosterone. Eur J Clin Pharmacol. 1992;42:641–644. - PubMed
-
- Barbarash RA, Bauman JL, Fischer JH, Kondos GT, Batenhorst RL. Near-total reduction in verapamil bioavailability by rifampin. Electrocardiographic correlates. Chest. 1988;94:954–959. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
