HSP101 functions as a specific translational regulatory protein whose activity is regulated by nutrient status
- PMID: 9784498
- PMCID: PMC317219
- DOI: 10.1101/gad.12.20.3236
HSP101 functions as a specific translational regulatory protein whose activity is regulated by nutrient status
Abstract
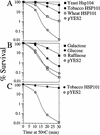
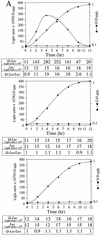
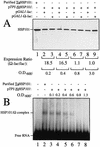
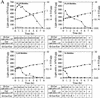
The 5' leader (Omega) of tobacco mosaic viral RNA functions as a translational enhancer. Sequence analysis of a 102-kD protein, identified previously as a specific Omega RNA-binding protein, revealed homology to the HSP101/HSP104/ClpB family of heat shock proteins and its expression in yeast complemented a thermotolerance defect caused by a deletion of the HSP104 gene. Up to a 50-fold increase in the translation of Omega-luc, but not luc mRNA was observed in yeast expressing the tobacco HSP101 whereas Omega failed to enhance translation in the absence of HSP101. Therefore, HSP101 and Omega comprise a two-component translational regulatory mechanism that can be recapitulated in yeast. Analysis of HSP101 function in yeast translation mutants suggested that the initiation factor (eIF) 3 and specifically one (TIF4632) of the two eIF4G proteins were required for the HSP101-mediated enhancement. The RNA-binding and translational regulatory activities of HSP101 were inactive in respiring cells or in cells subject to nutrient limitation, but its thermotolerance function remained unaffected. This is the first identification of a protein required for specific translational enhancement of capped mRNAs, the first report of a translational regulatory function for any heat-shock protein, and the first functional distinction between the two eIF4G proteins present in eukaryotes.
Figures












References
-
- Altmann M, Blum S, Pelletier J, Sonenberg N, Wilson TMA, Trachsel H. Translation initiation factor-dependent extracts from Saccharomyces cerevisiae. Biochim Biophys Acta. 1990a;1050:155–159. - PubMed
-
- Altmann M, Blum S, Wilson TMA, Trachsel H. The 5′-leader sequence of tobacco mosaic virus RNA mediates initiation-factor-4E-independent, but still initiation-factor-4A-independent translation in yeast extracts. Gene. 1990b;91:127–129. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
