Helicobacter pylori containing only cytoplasmic urease is susceptible to acid
- PMID: 9784504
- PMCID: PMC108630
- DOI: 10.1128/IAI.66.11.5060-5066.1998
Helicobacter pylori containing only cytoplasmic urease is susceptible to acid
Abstract
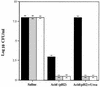
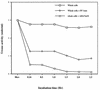
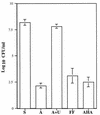
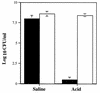
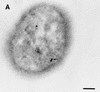
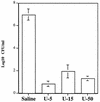
Helicobacter pylori, an important etiologic agent in a variety of gastroduodenal diseases, produces large amounts of urease as an essential colonization factor. We have demonstrated previously that urease is located within the cytoplasm and on the surface of H. pylori both in vivo and in stationary-phase culture. The purpose of the present study was to assess the relative contributions of cytoplasmic and surface-localized urease to the ability of H. pylori to survive exposure to acid in the presence of urea. Toward this end, we compared the acid resistance in vitro of H. pylori cells which possessed only cytoplasmic urease to that of bacteria which possessed both cytoplasmic and surface-localized or extracellular urease. Bacteria with only cytoplasmic urease activity were generated by using freshly subcultured bacteria or by treating repeatedly subcultured H. pylori with flurofamide (1 microM), a potent, but poorly diffusible urease inhibitor. H. pylori with cytoplasmic and surface-located urease activity survived in an acid environment when 5 mM urea was present. In contrast, H. pylori with only cytoplasmic urease shows significantly reduced survival when exposed to acid in the presence of 5 mM urea. Similarly, Escherichia coli SE5000 expressing H. pylori urease and the Ni2+ transport protein NixA, which expresses cytoplasmic urease activity at levels similar to those in wild-type H. pylori, survived minimally when exposed to acid in the presence of 5 to 50 mM urea. We conclude that cytoplasmic urease activity alone is not sufficient (although cytoplasmic urease activity is likely to be necessary) to allow survival of H. pylori in acid; the activity of surface-localized urease is essential for resistance of H. pylori to acid under the assay conditions used. Therefore, the mechanism whereby urease becomes associated with the surface of H. pylori, which involves release of the enzyme from bacteria due to autolysis followed by adsorption of the enzyme to the surface of intact bacteria ("altruistic autolysis"), is essential for survival of H. pylori in an acid environment. The ability of H. pylori to survive exposure to low pH is likely to depend on a combination of both cytoplasmic and surface-associated urease activities.
Figures


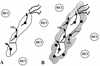
Similar articles
-
Structure, function and localization of Helicobacter pylori urease.Yale J Biol Med. 1998 Mar-Apr;71(2):63-73. Yale J Biol Med. 1998. PMID: 10378351 Free PMC article. Review.
-
Factors affecting growth and antibiotic susceptibility of Helicobacter pylori: effect of pH and urea on the survival of a wild-type strain and a urease-deficient mutant.J Med Microbiol. 1996 Jun;44(6):425-33. doi: 10.1099/00222615-44-6-425. J Med Microbiol. 1996. PMID: 8636959
-
Acid resistance of Helicobacter pylori depends on the UreI membrane protein and an inner membrane proton barrier.Mol Microbiol. 2000 Apr;36(1):141-52. doi: 10.1046/j.1365-2958.2000.01835.x. Mol Microbiol. 2000. PMID: 10760171
-
Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis.Infect Immun. 1996 Mar;64(3):905-12. doi: 10.1128/iai.64.3.905-912.1996. Infect Immun. 1996. PMID: 8641799 Free PMC article.
-
Acid survival of Helicobacter pylori: how does urease activity trigger cytoplasmic pH homeostasis?Trends Microbiol. 2002 Feb;10(2):70-4. doi: 10.1016/s0966-842x(01)02287-9. Trends Microbiol. 2002. PMID: 11827807 Review.
Cited by
-
Structure, function and localization of Helicobacter pylori urease.Yale J Biol Med. 1998 Mar-Apr;71(2):63-73. Yale J Biol Med. 1998. PMID: 10378351 Free PMC article. Review.
-
High extracellular levels of Mycobacterium tuberculosis glutamine synthetase and superoxide dismutase in actively growing cultures are due to high expression and extracellular stability rather than to a protein-specific export mechanism.Infect Immun. 2001 Oct;69(10):6348-63. doi: 10.1128/IAI.69.10.6348-6363.2001. Infect Immun. 2001. PMID: 11553579 Free PMC article.
-
Synthesis and Evaluation of 1,3,5-Triaryl-2-Pyrazoline Derivatives as Potent Dual Inhibitors of Urease and α-Glucosidase Together with Their Cytotoxic, Molecular Modeling and Drug-Likeness Studies.ACS Omega. 2022 Jan 20;7(4):3775-3795. doi: 10.1021/acsomega.1c06694. eCollection 2022 Feb 1. ACS Omega. 2022. PMID: 35128286 Free PMC article.
-
Nickel-responsive induction of urease expression in Helicobacter pylori is mediated at the transcriptional level.Infect Immun. 2001 Aug;69(8):4891-7. doi: 10.1128/IAI.69.8.4891-4897.2001. Infect Immun. 2001. PMID: 11447165 Free PMC article.
-
Identification of a novel penicillin-binding protein from Helicobacter pylori.J Bacteriol. 1999 Aug;181(16):5107-10. doi: 10.1128/JB.181.16.5107-5110.1999. J Bacteriol. 1999. PMID: 10438788 Free PMC article.
References
-
- Blaser M J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–727. - PubMed
-
- Bode G, Malfertheiner P, Lehnhardt G, Nilius M, Ditschuneit H. Ultrastructural localization of urease of Helicobacter pylori. Med Microbiol Immunol. 1993;182:233–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

