Lipoprotein release by bacteria: potential factor in bacterial pathogenesis
- PMID: 9784522
- PMCID: PMC108648
- DOI: 10.1128/IAI.66.11.5196-5201.1998
Lipoprotein release by bacteria: potential factor in bacterial pathogenesis
Abstract
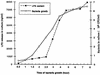
Lipoprotein (LP) is a major component of the outer membrane of bacteria in the family Enterobacteriaceae. LP induces proinflammatory cytokine production in macrophages and lethal shock in LPS-responsive and -nonresponsive mice. In this study, the release of LP from growing bacteria was investigated by immuno-dot blot analysis. An immuno-dot blot assay that could detect LP at levels as low as 100 ng/ml was developed. By using this assay, significant levels of LP were detected in culture supernatants of growing Escherichia coli cells. During mid-logarithmic growth, approximately 1 to 1.5 microgram of LP per ml was detected in culture supernatants from E. coli. In contrast, these culture supernatants contained 5 to 6 microgram/ml of lipopolysaccharide (LPS). LP release was not unique to E. coli. Salmonella typhimurium, Yersinia enterocolitica, and two pathogenic E. coli strains also released LP during in vitro growth. Treatment of bacteria with the antibiotic ceftazidime significantly enhanced LP release. Culture supernatants from 5-h cultures of E. coli were shown to induce in vitro production of interleukin-6 (IL-6) by macrophages obtained from LPS-nonresponsive C3H/HeJ mice. In contrast, culture supernatants from an E. coli LP-deletion mutant were significantly less efficient at inducing IL-6 production in C3H/HeJ macrophages. These results suggest, for the first time, that LP is released from growing bacteria and that this released LP may play an important role in the induction of cytokine production and pathologic changes associated with gram-negative bacterial infections.
Figures
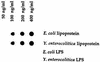






References
-
- Andersen B M, Solberg O. The endotoxin-liberating effect of antibiotics on meningococci in vitro. Acta Pathol Microbiol Scand Sect B. 1980;88:231–236. - PubMed
-
- Berkowitz F E, Vallabh P, Altman D I, Diamantes F, VanWyk H J, Stroucken J M. Jarisch-Herxheimer reaction in meningococcal meningitis. Am J Dis Child. 1983;137:599–603. - PubMed
-
- Bessler W G, Resch K, Hancock E, Hantke K. Induction of lymphocyte-proliferation and membrane changes by lipoprotein derivatives of the lipoprotein from the outer membrane of E. coli. Z Immunitaetsforsch. 1977;153:11–19. - PubMed
-
- Bessler W G, Ottenbreit B P. Studies on the mitogenic principal of lipoprotein from the outer membrane of E. coli. Biochem Biophys Res Commun. 1977;76:239–246. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

