Neisseria gonorrhoeae heme biosynthetic mutants utilize heme and hemoglobin as a heme source but fail to grow within epithelial cells
- PMID: 9784525
- PMCID: PMC108651
- DOI: 10.1128/IAI.66.11.5215-5223.1998
Neisseria gonorrhoeae heme biosynthetic mutants utilize heme and hemoglobin as a heme source but fail to grow within epithelial cells
Abstract
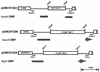
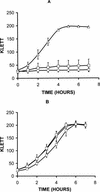
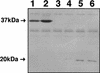
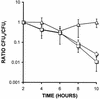
Many bacterial pathogens, including pathogenic neisseriae, can use heme as an iron source for growth. To study heme utilization by Neisseria gonorrhoeae, two heme biosynthetic mutants were constructed, one with a mutation in hemH (the gene encoding ferrochelatase) and one with a mutation in hemA (the gene encoding gamma-glutamyl tRNA reductase). The hemH mutant failed to grow without an exogenous supply of heme or hemoglobin, whereas the hemA mutant failed to grow unless heme, hemoglobin, or heme precursors were present. Growth of the mutants with hemoglobin required expression of the hemoglobin receptor (HpuAB) and was TonB dependent. However, growth with heme required neither HpuAB nor TonB. An fbpA mutant grew normally when either heme or hemoglobin was present in the medium. The heme biosynthetic mutants showed reduced intracellular survival, compared to the parent strain, within A-431 endocervical epithelial cell cultures. These studies demonstrate that in addition to synthesizing their own heme, N. gonorrhoeae strains are able to internalize and utilize exogenous heme independently of FbpA but appear unable to obtain heme from within epithelial cells for growth.
Figures



Similar articles
-
Role of the Haemophilus ducreyi Ton system in internalization of heme from hemoglobin.Infect Immun. 1998 Jan;66(1):151-60. doi: 10.1128/IAI.66.1.151-160.1998. Infect Immun. 1998. PMID: 9423852 Free PMC article.
-
Point mutations in HpuB enable gonococcal HpuA deletion mutants to grow on hemoglobin.J Bacteriol. 2002 Jan;184(2):420-6. doi: 10.1128/JB.184.2.420-426.2002. J Bacteriol. 2002. PMID: 11751818 Free PMC article.
-
Use of heme compounds as iron sources by pathogenic neisseriae requires the product of the hemO gene.J Bacteriol. 2000 Jan;182(2):439-47. doi: 10.1128/JB.182.2.439-447.2000. J Bacteriol. 2000. PMID: 10629191 Free PMC article.
-
Mechanisms of iron acquisition by the human pathogens Neisseria meningitidis and Neisseria gonorrhoeae.Front Biosci. 2003 Sep 1;8:d1186-218. doi: 10.2741/1133. Front Biosci. 2003. PMID: 12957813 Review.
-
Identification of receptor-mediated transferrin-iron uptake mechanism in Neisseria gonorrhoeae.Methods Enzymol. 1994;235:356-63. doi: 10.1016/0076-6879(94)35154-6. Methods Enzymol. 1994. PMID: 8057908 Review. No abstract available.
Cited by
-
The fbpABC operon is required for Ton-independent utilization of xenosiderophores by Neisseria gonorrhoeae strain FA19.Infect Immun. 2011 Jan;79(1):267-78. doi: 10.1128/IAI.00807-10. Epub 2010 Nov 1. Infect Immun. 2011. PMID: 21041493 Free PMC article.
-
The Neisseria meningitidis ZnuD zinc receptor contributes to interactions with epithelial cells and supports heme utilization when expressed in Escherichia coli.Infect Immun. 2012 Feb;80(2):657-67. doi: 10.1128/IAI.05208-11. Epub 2011 Nov 14. Infect Immun. 2012. PMID: 22083713 Free PMC article.
-
Investigation into the Antigenic Properties and Contributions to Growth in Blood of the Meningococcal Haemoglobin Receptors, HpuAB and HmbR.PLoS One. 2015 Jul 24;10(7):e0133855. doi: 10.1371/journal.pone.0133855. eCollection 2015. PLoS One. 2015. PMID: 26208277 Free PMC article.
-
The TB Structural Genomics Consortium: a decade of progress.Tuberculosis (Edinb). 2011 Mar;91(2):155-72. doi: 10.1016/j.tube.2010.11.009. Epub 2011 Jan 17. Tuberculosis (Edinb). 2011. PMID: 21247804 Free PMC article. Review.
-
Photoinactivation of Neisseria gonorrhoeae: A Paradigm-Changing Approach for Combating Antibiotic-Resistant Gonococcal Infection.J Infect Dis. 2019 Jul 31;220(5):873-881. doi: 10.1093/infdis/jiz018. J Infect Dis. 2019. PMID: 30629196 Free PMC article.
References
-
- Bagg A, Neilands J B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987;26:5471–5477. - PubMed
-
- Beale S I. Biosynthesis of hemes. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 731–748.
-
- Biswas G D, Anderson J E, Sparling P F. Cloning and functional characterization of Neisseria gonorrhoeae tonB, exbB and exbD genes. Mol Microbiol. 1997;24:169–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

