Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans
- PMID: 9784537
- PMCID: PMC108663
- DOI: 10.1128/IAI.66.11.5307-5313.1998
Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans
Abstract
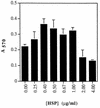
The subcellular locations, ultrastructure, and cytotoxic activity of the GroEL-like protein from Actinobacillus actinomycetemcomitans were investigated. Two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) clearly indicated that synthesis of the GroEL-like protein is substantially increased after a thermal shock. Analysis of the purified native GroEL-like protein by transmission electron microscopy revealed the typical 14-mer cylindrical molecule, which had a diameter of about 12 nm. A. actinomycetemcomitans cells grown at 35 degreesC and heat shocked at 43 degreesC were fractionated, and fractions were separated by SDS-PAGE and analyzed by Western immunoblotting using antibodies to GroEL- and DnaK-like proteins. The GroEL-like protein was found in both the soluble and membrane fractions, whereas the DnaK-like protein was mostly found in the cytoplasm. An increase in specific proteins, including the GroEL- and DnaK-like proteins, was found in heat-shocked cells. The subcellular localization of the GroEL-like protein was examined by immunoelectron microscopy of whole cells. More GroEL-like protein was detected in stressed cells than in unstressed cells, and most of it was found not directly associated with outer membranes but rather in extracellular material. The native GroEL-like protein was assessed for cytotoxic activities. The GroEL-like protein increased the proliferation of periodontal ligament epithelial cells at concentrations between 0.4 and 1.0 microgram/ml. The number of cells in the culture decreased significantly at higher concentrations. A cell viability assay using HaCaT epithelial cells indicated that the GroEL-like protein was strongly toxic for the cells. These studies suggest the extracellular nature of the GroEL-like protein and its putative role in disease initiation.
Figures




References
-
- Brunette D M, Melcher A H, Moe H K. Culture and origin of epithelium-like and fibroblast-like cells from porcine periodontal ligament explants and cell suspensions. Arch Oral Biol. 1976;21:393–400. - PubMed
-
- Ebersole J L, Cappelli D, Steffen M J. Longitudinal dynamics of infection and serum antibody in A. actinomycetemcomitans periodontitis. Oral Dis. 1995;1:129–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

