The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii
- PMID: 9784539
- PMCID: PMC108665
- DOI: 10.1128/IAI.66.11.5322-5328.1998
The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii
Abstract
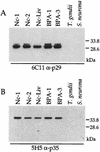
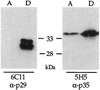
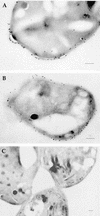
Neospora caninum is an apicomplexan parasite that is closely related to Toxoplasma gondii and has been found to be associated with neurological disorders in dogs and congenital infections and abortions in cattle. We have identified two surface proteins of 29 and 35 kDa (designated Ncp29 and Ncp35, respectively) from N. caninum tachyzoites that are the predominant antigens recognized by antisera from Neospora-infected animals. Monoclonal antibodies against Ncp29 and Ncp35 were used to analyze several independent and diverse N. caninum isolates; both antigens were recognized in all isolates, suggesting that they are well conserved. Localization studies and surface labeling with biotin demonstrated that Ncp29 and Ncp35 are membrane associated and displayed on the surface of the parasite. After treatment with phosphatidylinositol-specific phospholipase C, parasite lysates were analyzed with antibodies against the cross-reacting determinant of glycosylphosphatidylinositol anchors. Approximately six glycolipid-anchored surface proteins were identified, with the two most prominent corresponding to Ncp29 and Ncp35. Sequence comparisons of Ncp29 and Ncp35 with GenBank indicated that they are most similar to the T. gondii surface antigen 1 (SAG1) and surface antigen 1-related sequence 2 (SRS2), respectively. Consequently, Ncp29 has been designated NcSAG1 and Ncp35 has been designated NcSRS2. Both NcSAG1 and NcSRS2 contain a tandemly duplicated motif and 12 absolutely conserved cysteines which are also found in all of the SAG and SRS proteins of T. gondii. Maintenance of these motifs and the 12 cysteine residues suggests that these surface antigens share a similar secondary and tertiary structure that is presumably important for a conserved function that these antigens serve during infection.
Figures
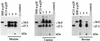


Similar articles
-
Differentiation of Neospora hughesi from Neospora caninum based on their immunodominant surface antigen, SAG1 and SRS2.Int J Parasitol. 1999 Oct;29(10):1575-82. doi: 10.1016/s0020-7519(99)00120-4. Int J Parasitol. 1999. PMID: 10608444
-
The major 36 kDa Neospora caninum tachyzoite surface protein is closely related to the major Toxoplasma gondii surface antigen.Mol Biochem Parasitol. 1998 Nov 30;97(1-2):97-108. doi: 10.1016/s0166-6851(98)00133-9. Mol Biochem Parasitol. 1998. PMID: 9879890
-
A4D12 monoclonal antibody recognizes a new linear epitope from SAG2A Toxoplasma gondii tachyzoites, identified by phage display bioselection.Immunobiology. 2010;215(1):26-37. doi: 10.1016/j.imbio.2009.01.008. Epub 2009 Mar 3. Immunobiology. 2010. PMID: 19261354
-
The antigenic composition of Neospora caninum.Int J Parasitol. 1999 Aug;29(8):1175-88. doi: 10.1016/s0020-7519(99)00085-5. Int J Parasitol. 1999. PMID: 10576569 Review.
-
Comparison of the major antigens of Neospora caninum and Toxoplasma gondii.Int J Parasitol. 1999 Oct;29(10):1489-96. doi: 10.1016/s0020-7519(99)00099-5. Int J Parasitol. 1999. PMID: 10608434 Review.
Cited by
-
Development of an Indirect ELISA Using Different Fragments of Recombinant Ncgra7 for Detection of Neospora caninum Infection in Cattle and Water Buffalo.Iran J Parasitol. 2015 Jan-Mar;10(1):69-77. Iran J Parasitol. 2015. PMID: 25904948 Free PMC article.
-
Determination of antigenic domain in GST fused major surface protein (Nc-p43) of Neospora caninum.Korean J Parasitol. 2001 Sep;39(3):241-6. doi: 10.3347/kjp.2001.39.3.241. Korean J Parasitol. 2001. PMID: 11590914 Free PMC article.
-
Selection of Neospora caninum antigens stimulating bovine CD4+ve T cell responses through immuno-potency screening and proteomic approaches.Vet Res. 2011 Aug 3;42(1):91. doi: 10.1186/1297-9716-42-91. Vet Res. 2011. PMID: 21813001 Free PMC article.
-
Neospora caninum: Structure and Fate of Multinucleated Complexes Induced by the Bumped Kinase Inhibitor BKI-1294.Pathogens. 2020 May 16;9(5):382. doi: 10.3390/pathogens9050382. Pathogens. 2020. PMID: 32429314 Free PMC article.
-
Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy.PLoS Pathog. 2012;8(3):e1002567. doi: 10.1371/journal.ppat.1002567. Epub 2012 Mar 22. PLoS Pathog. 2012. PMID: 22457617 Free PMC article.
References
-
- Ajioka J A, Boothroyd J C, Brunk B P, Hehl A, Hillier L, Manger I D, Overton G C, Marra M, Roos D, Wan K L. Gene discovery by EST sequencing in Toxoplasma gondii reveals sequences restricted to the Apicomplexa. Genome Res. 1998;8:18–28. - PubMed
-
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Anderson M L, Blanchard P C, Barr B C, Dubey J P, Hoffman R L, Conrad P A. Neospora-like protozoan infection as a major cause of abortion in California dairy cattle. J Am Vet Med Assoc. 1991;198:241–244. - PubMed
-
- Asai, T., D. K. Howe, K. Nakajima, T. Nozaki, T. Takeuchi, and L. D. Sibley. Neospora caninum: tachyzoites express a potent type-I nucleoside triphosphate hydrolase, but lack nucleoside diphosphate hydrolase activity. Exp. Parasitol., in press. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

