Pathways for potentiation of immunogenicity during adjuvant-assisted immunizations with Plasmodium falciparum major merozoite surface protein 1
- PMID: 9784540
- PMCID: PMC108666
- DOI: 10.1128/IAI.66.11.5329-5336.1998
Pathways for potentiation of immunogenicity during adjuvant-assisted immunizations with Plasmodium falciparum major merozoite surface protein 1
Abstract
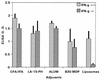
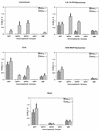
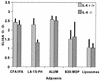
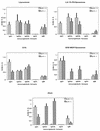
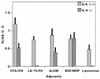
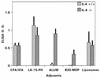
Vaccine adjuvants exert critical and unique influences on the quality of immune responses induced during active immunizations. We investigated the mechanisms of action of immunological adjuvants in terms of their requirements for cytokine-mediated pathways for adjuvanticity. Antibody responses potentiated by several adjuvants to a Plasmodium falciparum MSP1-19 (C-terminal 19-kDa processing fragment of MSP1) vaccine were studied in gamma interferon (IFN-gamma) or interleukin (IL-4) knockout mice. The levels of anti-MSP1-19 antibodies and the induction of Th1- and Th2-type antibodies were analyzed. Results revealed a spectrum of requirements for cytokine-mediated pathways in the potentiation of immunogenicity, and such requirements were influenced by interactions among individual components of the adjuvant formulations. One adjuvant strictly depended on IFN-gamma to induce appreciable levels of anti-MSP1-19 antibodies, while some formulations required IFN-gamma only for the induction of Th1-type antibodies. Other formulations induced exclusively Th2-type antibodies and were not affected by IFN-gamma knockout. There were three patterns of requirements for IL-4 by various adjuvants in the induction of Th2-type anti-MSP1-19 antibodies. Moreover, the induction of Th1-type anti-MSP1-19 antibodies by adjuvants showed two distinct patterns of regulation by IL-4. The utilization of an IL-4 regulated pathway(s) for the induction of Th2-type antibodies by the same adjuvant differed between mouse strains, suggesting that animal species variability in responses to vaccine adjuvants may be due, at least in part, to differences in the utilization of immune system pathways by an adjuvant among animal hosts.
Figures

References
-
- Aramaki Y, Suda H, Tsuchuya S. Interferon-gamma inductive effect of liposomes as an immunoadjuvant. Vaccine. 1995;13:1809–1814. - PubMed
-
- Banchereau J, Greene W C. Interleukin-4. In: Thomson A W, editor. The cytokine handbook. New York, N.Y: Academic Press; 1994. pp. 99–126.
-
- Brewer J M, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to Freund’s complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996;26:2062–2066. - PubMed
-
- Burghaus P A, Wellde B T, Hall T, Richards R L, Egan A F, Riley E M, Ballou W R, Holder A A. Immunization of Aotus nancymai with recombinant C terminus of Plasmodium falciparum merozoite surface protein 1 in liposomes and alum adjuvant does not induce protection against a challenge infection. Infect Immun. 1996;64:3614–3619. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

