Mechanisms involved in the pathogenesis of sepsis are not necessarily reflected by in vitro cell activation studies
- PMID: 9784546
- PMCID: PMC108672
- DOI: 10.1128/IAI.66.11.5372-5378.1998
Mechanisms involved in the pathogenesis of sepsis are not necessarily reflected by in vitro cell activation studies
Abstract
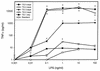
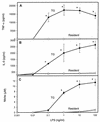
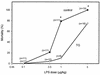
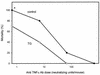
It is thought that lipopolysaccharide (LPS) from gram-negative bacteria contributes significantly to the pathogenesis of septic shock. In vitro studies to address the mechanisms involved in this process have often investigated human monocytes or mouse macrophages, since these cells produce many of the mediators found in septic patients. Targeting of these mediators, especially tumor necrosis factor alpha (TNF-alpha), has been pursued as a means of reducing mortality in sepsis. Two experimental approaches were designed to test the assumption that in vitro studies with macrophages accurately predict in vivo mechanisms of LPS pathogenesis. In the first approach, advantage was taken of the fact that on consecutive days after injection of thioglycolate into mice, increased numbers of macrophages could be harvested from the peritoneum. These cells manifested markedly enhanced levels of in vitro TNF-alpha, interleukin 6 (IL-6), and nitric oxide production in response to LPS. In D-galactosamine-sensitized mice, however, thioglycolate treatment significantly decreased mortality due to LPS, as well as levels of circulating TNF-alpha and IL-6. Anti-TNF-alpha treatment confirmed this cytokine's role in the observed lethality. In a second experimental approach, we compared the mouse macrophage-stimulating potencies of different LPS preparations with their lethalities to mice. In these studies, the in vitro macrophage-stimulating profiles presented by rough-LPS and smooth-LPS preparations were the reverse of their relative lethal potencies in vivo. In conclusion, peritoneal macrophages appear not to be the major cells responsible for the overall host response during endotoxic shock. These findings underscore the importance of verifying the correlation of in vivo systems with in vitro systems when attributing specific functions to a cell type.
Figures


References
-
- Abraham E, Wunderink R, Silverman H, Perl T M, Nasraway S, Levy H, Bone R, Wenzel R P, Balk R, Allred R, et al. Efficacy and safety of monoclonal antibodies to tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha Mab sepsis group. JAMA. 1997;273:934–941. - PubMed
-
- Amura C R, Chen L C, Hirohashi N, Lei M G, Morrison D C. Two functionally independent pathways for the LPS-dependent activation of mouse peritoneal macrophages. J Immunol. 1997;159:5079–5083. - PubMed
-
- Amura C R, Fontan P A, Sanjuan N, Sordelli D O. The effect of treatment with interleukin-1 and tumor necrosis factor on Pseudomonas aeruginosa lung infection in a granulocytopenic mouse model. Clin Immunol Immunopathol. 1994;73:261–266. - PubMed
-
- Beutler B, Milsark I W, Cerami A. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effects of endotoxin. Science. 1985;229:869–871. - PubMed
-
- Bone R C. Sepsis syndrome. New insights into its pathogenesis and treatment. Infect Dis Clin N Am. 1991;5:793–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

