DNA and a CpG oligonucleotide derived from Babesia bovis are mitogenic for bovine B cells
- PMID: 9784553
- PMCID: PMC108679
- DOI: 10.1128/IAI.66.11.5423-5432.1998
DNA and a CpG oligonucleotide derived from Babesia bovis are mitogenic for bovine B cells
Abstract
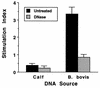
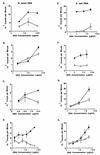
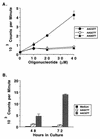
DNAs from bacteria and variety of nonvertebrate organisms, including nematodes, mollusks, yeasts, and insects, cause polyclonal activation of murine B lymphocytes. Similar studies have not been reported for bovine B cells, and to date no studies have reported mitogenic properties of protozoal DNA for any species. However, we and others have observed that protozoal parasite antigens can induce the proliferation of lymphocytes from nonexposed donors. Extending these studies, we now show that the mitogenic property of protozoal antigen preparations is in part attributable to parasite DNA and that Babesia bovis DNA is directly mitogenic for bovine B cells. DNase treatment of B. bovis extracts abrogated B. bovis-induced proliferation of peripheral blood mononuclear cells from nonexposed cattle. Like DNAs from other organisms that were mitogenic for murine B cells, B. bovis DNA is largely nonmethylated and induced a dose-dependent proliferation of bovine B cells, which was reduced upon methylation. Furthermore, B. bovis and E. coli DNAs enhanced immunoglobulin secretion by cultured B cells, inducing moderate increases in immunoglobulin G1 and stronger increases in immunoglobulin G2. Because certain nonmethylated CpG motifs present in bacterial DNA are known to stimulate proliferation of murine and human B cells, an 11-kb fragment of B. bovis DNA was analyzed for CG dinucleotide content and for the presence of known immunostimulatory sequences (ISS) centered on a CG motif. The frequency of CG dinucleotides was approximately one-half of the expected frequency, and several CpG hexameric sequences with known activity for murine B cells were identified. An oligodeoxynucleotide containing one of these ISS (AACGTT), which is present within the rhoptry-associated protein-1 (rap-1) open reading frame, was shown to stimulate B-cell proliferation. These ISS may be involved in host immune modulation during protozoal infection and may be useful as vaccine adjuvants.
Figures

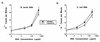

References
-
- Ballas Z K, Rasmussen W L, Krieg A M. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840–1845. - PubMed
-
- Bird A P. CpG islands as gene markers in the vertebrate nucleus. Trends Genet. 1987;3:342–347.
-
- Branda R F, Moore A L, Mathews L, McCormack J J, Zon G. Immune stimulation by an antisense oligomer complementary to the rev gene of HIV-1. Biochem Pharmacol. 1993;45:2037–2043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

