Autoregulation of periodontal ligament cell phenotype and functions by transforming growth factor-beta1
- PMID: 9786634
- PMCID: PMC4950996
- DOI: 10.1177/00220345980770100501
Autoregulation of periodontal ligament cell phenotype and functions by transforming growth factor-beta1
Abstract
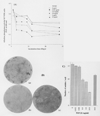
During orthodontic tooth movement, mechanical forces acting on periodontal ligament (PDL) cells induce the synthesis of mediators which alter the growth, differentiation, and secretory functions of cells of the PDL. Since the cells of the PDL represent a heterogeneous population, we examined mechanically stress-induced cytokine profiles in three separate clones of human osteoblast-like PDL cells. Of the four pro-inflammatory cytokines investigated, only IL-6 and TGF-beta1 were up-regulated in response to mechanical stress. However, the expression of other pro-inflammatory cytokines such as IL-1 beta, TNF-alpha, or IL-8 was not observed. To understand the consequences of the increase in TGF-beta1 expression following mechanical stress, we examined the effect of TGF-beta1 on PDL cell phenotype and functions. TGF-beta1 was mitogenic to PDL cells at concentrations between 0.4 and 10 ng/mL. Furthermore, TGF-beta1 down-regulated the osteoblast-like phenotype of PDL cells, i.e., alkaline phosphatase activity, calcium phosphate nodule formation, expression of osteocalcin, and TGF-beta1, in a dose-dependent manner. Although initially TGF-beta1 induced expression of type I collagen mRNA, prolonged exposure to TGF-beta1 down-regulated the ability of PDL cells to express type I collagen mRNA. Our results further show that, within 4 hrs, exogenously applied TGF-beta1 down-regulated IL-6 expression in a dose-dependent manner, and this inhibition was sustained over a six-day period. In summary, the data suggest that mechanically stress-induced TGF-beta1 expression may be a physiological mechanism to induce mitogenesis in PDL cells while down-regulating its osteoblast-like features and simultaneously reducing the IL-6-induced bone resorption.
Figures







References
-
- Arceo N, Sauk JJ, Moehring J, Foster RA, Somerman MJ. Human periodontal cells initiate mineral like nodules in vitro. J Periodont Res. 1991;62:499–503. - PubMed
-
- Banes AJ, Gilbert J, Taylor D, Monbureau O. A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J Cell Sci. 1985;75:35–42. - PubMed
-
- Binderman I, Zor U, Kaye AM, Shimshoni Z, Harell A, Somjen D. The transduction of mechanical force into biochemical events in bone cells may involve activation of phospholipase A2. Calcif Tissue Int. 1988;42:261–266. - PubMed
-
- Bonewald LF, Mundy GR. Role of transforming growth factor-β on bone remodeling. Clin Orthop Rel Res. 1990;250:261–276. - PubMed
-
- Breen EC, Ignotz RA, McCabe L, Stein JL, Stein GS, Lian JB. TGF beta alters growth and differentiation related gene expression in proliferating osteoblasts in vitro, preventing development of the mature bone phenotype. J Cell Physiol. 1994;160:323–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

