The membrane transport factor TAP/p115 cycles between the Golgi and earlier secretory compartments and contains distinct domains required for its localization and function
- PMID: 9786945
- PMCID: PMC2132831
- DOI: 10.1083/jcb.143.2.319
The membrane transport factor TAP/p115 cycles between the Golgi and earlier secretory compartments and contains distinct domains required for its localization and function
Abstract
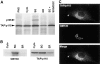
The mammalian protein TAP/p115 and its yeast homologue Uso1p have an essential role in membrane traffic (Nakajima et al., 1991; Waters et al., 1992; Sztul et al., 1993; Rabouille et al.; 1995). To inquire into the site and mechanism of TAP/p115 action, we aimed to localize it and to identify domains required for its function. We show that in interphase cells, TAP/p115 localizes predominantly to the Golgi and to peripheral structures that represent vesicular tubular clusters (VTCs) involved in ER to Golgi transport. Using BFA/ nocodazole treatments we confirm that TAP/p115 is present on ER to Golgi transport intermediates. TAP/ p115 redistributes to peripheral structures containing ERGIC-53 during a 15 degreesC treatment, suggesting that it is a cycling protein. Within the Golgi, TAP/p115 is associated with pleiomorphic structures on the cis side of the cis-Golgi cisterna and the cis-most cisterna, but is not detected in more distal compartments of the Golgi. TAP/p115 binds the cis-Golgi protein GM130, and the COOH-terminal acidic domain of TAP/p115 is required for this interaction. TAP/p115 interaction with GM130 occurs only in the Golgi and is not required for TAP/p115 association with peripheral VTCs. To examine whether interaction with GM130 is required to recruit TAP/p115 to the Golgi, TAP/p115 mutants lacking the acidic domain were expressed and localized in transfected cells. Mutants lacking the GM130-binding domain showed normal Golgi localization, indicating that TAP/p115 is recruited to the Golgi independently of its ability to bind GM130. Such mutants were also able to associate with peripheral VTCs. Interestingly, TAP/p115 mutants containing the GM130-binding domain but lacking portions of the NH2-terminal region were restricted from the Golgi and localized to the ER. The COOH-terminal domain required for GM130 binding and the NH2-terminal region required for Golgi localization appear functionally relevant since expression of TAP/p115 mutants lacking either of these domains leads to loss of normal Golgi morphology.
Figures




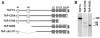



References
-
- Ansorge W. Fast and sensitive detection of protein and DNA bands by treatment with potassium permanganate. J Biochem Biophys Methods. 1985;11:13–20. - PubMed
-
- Bajjalieh SM, Scheller RH. The biochemistry of neurotransmitter secretion. J Biol Chem. 1995;270:1971–1974. - PubMed
-
- Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

