A human dynamin-related protein controls the distribution of mitochondria
- PMID: 9786947
- PMCID: PMC2132828
- DOI: 10.1083/jcb.143.2.351
A human dynamin-related protein controls the distribution of mitochondria
Abstract
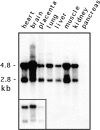
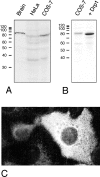
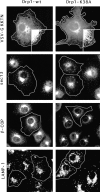
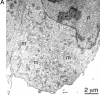
Mitochondria exist as a dynamic tubular network with projections that move, break, and reseal in response to local environmental changes. We present evidence that a human dynamin-related protein (Drp1) is specifically required to establish this morphology. Drp1 is a GTPase with a domain structure similar to that of other dynamin family members. To identify the function of Drp1, we transiently transfected cells with mutant Drp1. A mutation in the GTPase domain caused profound alterations in mitochondrial morphology. The tubular projections normally present in wild-type cells were retracted into large perinuclear aggregates in cells expressing mutant Drp1. The morphology of other organelles was unaffected by mutant Drp1. There was also no effect of mutant Drp1 on the transport functions of the secretory and endocytic pathways. By EM, the mitochondrial aggregates found in cells that were transfected with mutant Drp1 appear as clusters of tubules rather than a large mass of coalescing membrane. We propose that Drp1 is important for distributing mitochondrial tubules throughout the cell. The function of this new dynamin-related protein in organelle morphology represents a novel role for a member of the dynamin family of proteins.
Figures





References
-
- Arnheiter H, Meier E. MX proteins: antiviral proteins by chance or by necessity? . New Biol. 1990;2:851–857. - PubMed
-
- Bereiter-Hahn J, Voth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Techn. 1994;27:198–219. - PubMed
-
- Berger KH, Yaffe MP. Mitochondrial distribution and inheritance. Experientia. 1996;52:1111–1116. - PubMed
-
- Cascarano J, Chambers PA, Schwartz E, Poorkaj P, Gondo RE. Organellar clusters formed by mitochondrial-rough endoplasmic reticulum associations: an ordered arrangement of mitochondria in hepatocytes. Hepatology. 1995;22:837–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

