A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts
- PMID: 9786950
- PMCID: PMC2132845
- DOI: 10.1083/jcb.143.2.391
A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts
Abstract
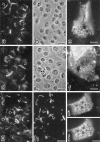
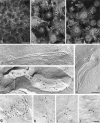
Three integral membrane proteins, clau- din-1, -2, and occludin, are known to be components of tight junction (TJ) strands. To examine their ability to form TJ strands, their cDNAs were introduced into mouse L fibroblasts lacking TJs. Immunofluorescence microscopy revealed that both FLAG-tagged claudin-1 and -2 were highly concentrated at cell contact sites as planes through a homophilic interaction. In freeze-fracture replicas of these contact sites, well-developed networks of strands were identified that were similar to TJ strand networks in situ and were specifically labeled with anti-FLAG mAb. In glutaraldehyde-fixed samples, claudin-1-induced strands were largely associated with the protoplasmic (P) face as mostly continuous structures, whereas claudin-2-induced strands were discontinuous at the P face with complementary grooves at the extracellular (E) face which were occupied by chains of particles. Although occludin was also concentrated at cell contact sites as dots through its homophilic interaction, freeze-fracture replicas identified only a small number of short strands that were labeled with anti-occludin mAb. However, when occludin was cotransfected with claudin-1, it was concentrated at cell contact sites as planes to be incorporated into well- developed claudin-1-based strands. These findings suggested that claudin-1 and -2 are mainly responsible for TJ strand formation, and that occludin is an accessory protein in some function of TJ strands.
Figures




References
-
- Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. - PubMed
-
- Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

