Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis
- PMID: 9786961
- PMCID: PMC2132838
- DOI: 10.1083/jcb.143.2.533
Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis
Abstract
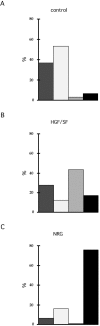
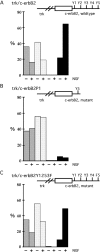
We have established a cell culture system that reproduces morphogenic processes in the developing mammary gland. EpH4 mouse mammary epithelial cells cultured in matrigel form branched tubules in the presence of hepatocyte growth factor/scatter factor (HGF/SF), the ligand of the c-met tyrosine kinase receptor. In contrast, alveolar structures are formed in the presence of neuregulin, a ligand of c-erbB tyrosine kinase receptors. These distinct morphogenic responses can also be observed with selected human mammary carcinoma tissue in explant culture. HGF/SF-induced branching was abrogated by the PI3 kinase inhibitors wortmannin and LY294002. In contrast, neuregulin- induced alveolar morphogenesis was inhibited by the MAPK kinase inhibitor PD98059. The c-met-mediated response could also be evoked by transfection of a c-met specific substrate, Gab1, which can activate the PI3 kinase pathway. An activated hybrid receptor that contained the intracellular domain of c-erbB2 receptor suffices to induce alveolar morphogenesis, and was observed in the presence of tyrosine residues Y1028, Y1144, Y1201, and Y1226/27 in the substrate-binding domain of c-erbB2. Our data demonstrate that c-met and c-erbB2 signaling elicit distinct morphogenic programs in mammary epithelial cells: formation of branched tubules relies on a pathway involving PI3 kinase, whereas alveolar morphogenesis requires MAPK kinase.
Figures


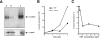


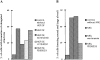




Similar articles
-
Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells.J Cell Biol. 1995 Dec;131(6 Pt 1):1573-86. doi: 10.1083/jcb.131.6.1573. J Cell Biol. 1995. PMID: 8522613 Free PMC article.
-
Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland.J Cell Biol. 1995 Oct;131(1):215-26. doi: 10.1083/jcb.131.1.215. J Cell Biol. 1995. PMID: 7559778 Free PMC article.
-
Role of HGF/SF and c-Met in morphogenesis and metastasis of epithelial cells.Ciba Found Symp. 1997;212:230-40; discussion 240-6. doi: 10.1002/9780470515457.ch15. Ciba Found Symp. 1997. PMID: 9524774 Review.
-
Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase.J Biol Chem. 1995 Nov 17;270(46):27780-7. doi: 10.1074/jbc.270.46.27780. J Biol Chem. 1995. PMID: 7499247
-
Hepatocyte growth factor and neuregulin in mammary gland cell morphogenesis.Adv Exp Med Biol. 2000;480:9-18. doi: 10.1007/0-306-46832-8_2. Adv Exp Med Biol. 2000. PMID: 10959405 Review.
Cited by
-
Investigating the interplay between the mir-183/182/96 cluster and the adherens junction pathway in early-stage breast cancer.Sci Rep. 2024 Oct 21;14(1):24711. doi: 10.1038/s41598-024-73632-0. Sci Rep. 2024. PMID: 39433788 Free PMC article.
-
Aberrant c-erbB2 expression in cell clusters overlying focally disrupted breast myoepithelial cell layers: a trigger or sign for emergence of more aggressive cell clones?Int J Biol Sci. 2008 Aug 16;4(5):259-69. doi: 10.7150/ijbs.4.259. Int J Biol Sci. 2008. PMID: 18726004 Free PMC article.
-
The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression.EMBO J. 2000 May 2;19(9):2024-33. doi: 10.1093/emboj/19.9.2024. EMBO J. 2000. PMID: 10790369 Free PMC article.
-
Gab1 mediates hepatocyte growth factor-stimulated mitogenicity and morphogenesis in multipotent myeloid cells.J Cell Biochem. 2010 Oct 1;111(2):310-21. doi: 10.1002/jcb.22695. J Cell Biochem. 2010. PMID: 20506405 Free PMC article.
-
Three-dimensional cultures of mouse mammary epithelial cells.Methods Mol Biol. 2013;945:221-50. doi: 10.1007/978-1-62703-125-7_14. Methods Mol Biol. 2013. PMID: 23097110 Free PMC article.
References
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Bacus SS, Gudkov AV, Zelnick CR, Chin D, Stern R, Stancovski I, Peles E, Ben-Baruch N, Farbstein H, Lupu R, et al. Neu differentiation factor (heregulin) induces expression of intercellular adhesion molecule 1: implications for mammary tumors. Cancer Res. 1993;53:5251–5261. - PubMed
-
- Berdichevsky F, Alford D, D'souza B, Taylor-Papadimitriou J. Branching morphogenesis of human mammary epithelial cells in collagen gels. J Cell Sci. 1994;107:3557–3568. - PubMed
-
- Binas B, Spitzer E, Zschiesche W, Erdmann B, Kurtz A, Müller T, Niemann C, Blenau W, Grosse R. Hormonal induction of functional differentiation and mammary-derived growth inhibitor expression in cultured mouse mammary explants. Cell Dev Biol. 1992;18:625–634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

