cAMP-dependent long-term potentiation of nitric oxide release from cerebellar parallel fibers in rats
- PMID: 9786963
- PMCID: PMC6793512
- DOI: 10.1523/JNEUROSCI.18-21-08551.1998
cAMP-dependent long-term potentiation of nitric oxide release from cerebellar parallel fibers in rats
Abstract
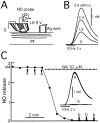
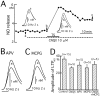
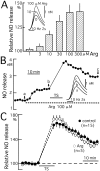
Nitric Oxide (NO) is released from parallel fibers (PFs) after PF stimulation. NO-cGMP signaling is essential for long-term depression (LTD) in cerebellar PF-Purkinje cell synapses, which also exhibit presynaptic long-term potentiation (LTP) after tetanic PF stimulation. This LTP is dependent on cAMP but not NO-cGMP signaling. In this study, we analyzed long-term changes of NO release from PFs in rat cerebellar slices using electrochemical NO probes. Repetitive PF stimulation at 10 Hz for 2 sec elicited a transient increase in NO concentration (2.2 +/- 0.1 nM; mean +/- SEM; n = 116). This NO release exhibited long-term potentiation (LTPNO) by 36 +/- 3% (n = 15) after tetanic PF stimulation. Induction of LTPNO was not affected by Glu receptor antagonists. NO release from PFs was also potentiated by L-Arg (ARG) (100 microM), forskolin (50 microM), and 8-bromo-cAMP (Br-cAMP) (1 mM) but not by 1,9-dideoxyforskolin (50 microM), a biologically inactive analog of forskolin. The potentiation induced by forskolin was significantly suppressed by H89 (10 microM), a blocker of cAMP-dependent protein kinase. The potentiation induced by forskolin, but not that induced by Arg, interfered with LTPNO. H89 (10 microM) and KT5720 (1 microM), another blocker of cAMP-dependent protein kinase, but not KT5823 (300 nM), a blocker of cGMP-dependent protein kinase, significantly suppressed LTPNO. These data indicate that neural NO release is under activity-dependent control, just as synaptic transmitter release is. LTPNO might play a role in cross talk between presynaptic and postsynaptic plasticity by facilitating NO-cGMP-dependent postsynaptic LTD after induction of cAMP-dependent presynaptic LTP and LTPNO.
Figures



Similar articles
-
Nitric oxide is required for the induction and heterosynaptic spread of long-term potentiation in rat cerebellar slices.J Physiol. 2001 Sep 15;535(Pt 3):825-39. doi: 10.1111/j.1469-7793.2001.t01-1-00825.x. J Physiol. 2001. PMID: 11559778 Free PMC article.
-
GABA activity mediating cytosolic Ca2+ rises in developing neurons is modulated by cAMP-dependent signal transduction.J Neurosci. 1997 Jun 15;17(12):4785-99. doi: 10.1523/JNEUROSCI.17-12-04785.1997. J Neurosci. 1997. PMID: 9169537 Free PMC article.
-
NMDA receptor-mediated stimulation of rat cerebellar nitric oxide formation is modulated by cyclic AMP.Eur J Pharmacol. 1994 Jan 1;266(1):63-6. doi: 10.1016/0922-4106(94)90210-0. Eur J Pharmacol. 1994. PMID: 8137885
-
Forskolin and 3-isobutyl-1-methylxanthine increase basal and sodium nitroprusside-elevated cyclic GMP levels in adult guinea-pig cerebellar slices.J Neurochem. 1994 Jun;62(6):2212-8. doi: 10.1046/j.1471-4159.1994.62062212.x. J Neurochem. 1994. PMID: 7514648
-
Nitric oxide and carbon monoxide as possible retrograde messengers in hippocampal long-term potentiation.J Neurobiol. 1994 Jun;25(6):652-65. doi: 10.1002/neu.480250607. J Neurobiol. 1994. PMID: 8071665 Review.
Cited by
-
Overexpression of copper/zinc superoxide dismutase in transgenic mice protects against neuronal cell death after transient focal ischemia by blocking activation of the Bad cell death signaling pathway.J Neurosci. 2003 Mar 1;23(5):1710-8. doi: 10.1523/JNEUROSCI.23-05-01710.2003. J Neurosci. 2003. PMID: 12629175 Free PMC article.
-
Nitric oxide is required for the induction and heterosynaptic spread of long-term potentiation in rat cerebellar slices.J Physiol. 2001 Sep 15;535(Pt 3):825-39. doi: 10.1111/j.1469-7793.2001.t01-1-00825.x. J Physiol. 2001. PMID: 11559778 Free PMC article.
-
Synapses between parallel fibres and stellate cells express long-term changes in synaptic efficacy in rat cerebellum.J Physiol. 2004 Feb 1;554(Pt 3):707-20. doi: 10.1113/jphysiol.2003.055871. Epub 2003 Nov 14. J Physiol. 2004. PMID: 14617674 Free PMC article.
-
cAMP-EPAC-PKCε-RIM1α signaling regulates presynaptic long-term potentiation and motor learning.Elife. 2023 Apr 26;12:e80875. doi: 10.7554/eLife.80875. Elife. 2023. PMID: 37159499 Free PMC article.
-
Modulation of neurotransmitter release by the second messenger-activated protein kinases: implications for presynaptic plasticity.Pharmacol Ther. 2005 Jan;105(1):69-84. doi: 10.1016/j.pharmthera.2004.10.012. Pharmacol Ther. 2005. PMID: 15626456 Free PMC article. Review.
References
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Aoki C, Fenstemaker S, Lubin M, Go CG. Nitric oxide synthase in the visual cortex of monocular monkeys as revealed by light and electron microscopic immunocytochemistry. Brain Res. 1993;620:97–113. - PubMed
-
- Aoki E, Semba R, Mikoshiba K, Kashiwamata S. Predominant localization in glial cells of free l-arginine. Immunocytochemical evidence. Brain Res. 1991;547:190–192. - PubMed
-
- Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD. Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell. 1996;87:1025–1035. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
