alpha-conotoxin AuIB selectively blocks alpha3 beta4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release
- PMID: 9786965
- PMCID: PMC6793526
- DOI: 10.1523/JNEUROSCI.18-21-08571.1998
alpha-conotoxin AuIB selectively blocks alpha3 beta4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release
Abstract
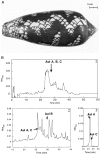
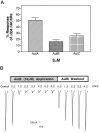
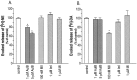
Neuronal nicotinic acetylcholine receptors (nAChRs) with putative alpha3 beta4-subunits have been implicated in the mediation of signaling in various systems, including ganglionic transmission peripherally and nicotine-evoked neurotransmitter release centrally. However, progress in the characterization of these receptors has been hampered by a lack of alpha3 beta4-selective ligands. In this report, we describe the purification and characterization of an alpha3 beta4 nAChR antagonist, alpha-conotoxin AuIB, from the venom of the "court cone," Conus aulicus. We also describe the total chemical synthesis of this and two related peptides that were also isolated from the venom. alpha-Conotoxin AuIB blocks alpha3 beta4 nAChRs expressed in Xenopus oocytes with an IC50 of 0.75 microM, a kon of 1.4 x 10(6) min-1 M-1, a koff of 0.48 min-1, and a Kd of 0.5 microM. Furthermore, alpha-conotoxin AuIB blocks the alpha3 beta4 receptor with >100-fold higher potency than other receptor subunit combinations, including alpha2 beta2, alpha2 beta4, alpha3 beta2, alpha4 beta2, alpha4 beta4, and alpha1 beta1 gamma delta. Thus, AuIB is a novel, selective probe for alpha3 beta4 nAChRs. AuIB (1-5 microM) blocks 20-35% of the nicotine-stimulated norepinephrine release from rat hippocampal synaptosomes, whereas nicotine-evoked dopamine release from striatal synaptosomes is not affected. Conversely, the alpha3 beta2-specific alpha-conotoxin MII (100 nM) blocks 33% of striatal dopamine release but not hippocampal norepinephrine release. This suggests that in the respective systems, alpha3 beta4-containing nAChRs mediate norepinephrine release, whereas alpha3 beta2-containing receptors mediate dopamine release.
Figures


References
-
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new α-conotoxin which targets α3β2 nicotinic acetylcholine receptors. J Biol Chem. 1996a;271:7522–7528. - PubMed
-
- Cartier GE, Yoshikami D, Luo S, Olivera BM, McIntosh JM. α-Conotoxin MII (α-CTx-MII) interaction with neuronal nicotinic acetylcholine receptors. Soc Neurosci Abstr. 1996b;22:268.
-
- Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem. 1995;270:4424–4431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
