The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties
- PMID: 9786970
- PMCID: PMC6793533
- DOI: 10.1523/JNEUROSCI.18-21-08625.1998
The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties
Abstract
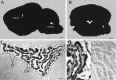
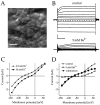
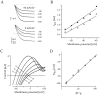
Rat and human cDNAs were isolated that both encoded a 360 amino acid polypeptide with a tertiary structure typical of inwardly rectifying K+ channel (Kir) subunits. The new proteins, termed Kir7.1, were <37% identical to other Kir subunits and showed various unique residues at conserved sites, particularly near the pore region. High levels of Kir7.1 transcripts were detected in rat brain, lung, kidney, and testis. In situ hybridization of rat brain sections demonstrated that Kir7.1 mRNA was absent from neurons and glia but strongly expressed in the secretory epithelial cells of the choroid plexus (as confirmed by in situ patch-clamp measurements). In cRNA-injected Xenopus oocytes Kir7.1 generated macroscopic Kir currents that showed a very shallow dependence on external K+ ([K+]e), which is in marked contrast to all other Kir channels. At a holding potential of -100 mV, the inward current through Kir7.1 averaged -3.8 +/- 1.04 microA with 2 mM [K+]e and -4.82 +/- 1.87 microA with 96 mM [K+]e. Kir7.1 has a methionine at position 125 in the pore region where other Kir channels have an arginine. When this residue was replaced by the conserved arginine in mutant Kir7.1 channels, the pronounced dependence of K+ permeability on [K+]e, characteristic for other Kir channels, was restored and the Ba2+ sensitivity was increased by a factor of approximately 25 (Ki = 27 microM). These findings support the important role of this site in the regulation of K+ permeability in Kir channels by extracellular cations.
Figures






References
-
- Armstrong C. The vision of the pore. Science. 1998;280:56–57. - PubMed
-
- Baumann A, Krah-Jentgens I, Müller R, Müller-Holtkamp F, Seidel R, Kecskemethy N, Casal J, Ferrus A, Pongs O. Molecular organization of the maternal effect region of the Shaker complex of Drosophila: characterization of an IA channel transcript with homology to vertebrate Na+ channel. EMBO J. 1987;6:3419–3429. - PMC - PubMed
-
- Cohen NA, Brenman JE, Snyder SH, Bredt DS. Binding of the inward rectifier K+ channel Kir2.3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996;17:759–767. - PubMed
-
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, Mackinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
