The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission
- PMID: 9786972
- PMCID: PMC6793563
- DOI: 10.1523/JNEUROSCI.18-21-08648.1998
The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission
Abstract
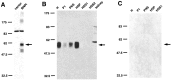
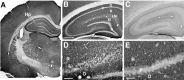
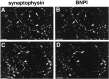
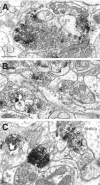
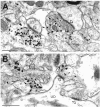
Molecular cloning has recently identified a vertebrate brain-specific Na+-dependent inorganic phosphate transporter (BNPI). BNPI has strong sequence similarity to EAT-4, a Caenorhabditis elegans protein implicated in glutamatergic transmission. To characterize the physiological role of BNPI, we have generated an antibody to the protein. Immunocytochemistry of rat brain sections shows a light microscopic pattern that is suggestive of reactivity in nerve terminals. Excitatory projections are labeled prominently, and ultrastructural analysis confirms that BNPI localizes almost exclusively to terminals forming asymmetric excitatory-type synapses. Although BNPI depends on a Na+ gradient and presumably functions at the plasma membrane, both electron microscopy and biochemical fractionation show that BNPI associates preferentially with the membranes of small synaptic vesicles. The results provide anatomic evidence of a specific presynaptic role for BNPI in glutamatergic neurotransmission, consistent with the phenotype of eat-4 mutants. Because an enzyme known as the phosphate-activated glutaminase produces glutamate for release as a neurotransmitter, BNPI may augment excitatory transmission by increasing cytoplasmic phosphate concentrations within the nerve terminal and hence increasing glutamate synthesis. Expression of BNPI on synaptic vesicles suggests a mechanism for neural activity to regulate the function of BNPI.
Figures




References
-
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus. I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. - PubMed
-
- Aoki C, Kaneko T, Starr A, Pickel VM. Identification of mitochondrial and non-mitochondrial glutaminase within select neurons and glia of rat forebrain by electron microscopic immunocytochemistry. J Neurosci Res. 1991;28:531–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
