Differences in Ca2+ channels governing generation of miniature and evoked excitatory synaptic currents in spinal laminae I and II
- PMID: 9786981
- PMCID: PMC6793560
- DOI: 10.1523/JNEUROSCI.18-21-08740.1998
Differences in Ca2+ channels governing generation of miniature and evoked excitatory synaptic currents in spinal laminae I and II
Abstract
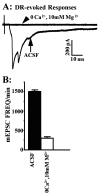
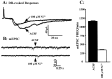
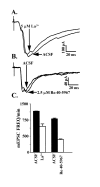
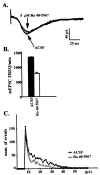
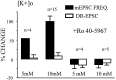
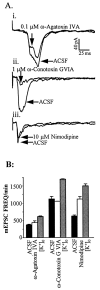
Many neurons of spinal laminae I and II, a region concerned with pain and other somatosensory mechanisms, display frequent miniature "spontaneous" EPSCs (mEPSCs). In a number of instances, mEPSCs occur often enough to influence neuronal excitability. To compare generation of mEPSCs to EPSCs evoked by dorsal root stimulation (DR-EPSCs), various agents affecting neuronal activity and Ca2+ channels were applied to in vitro slice preparations of rodent spinal cord during tight-seal, whole-cell, voltage-clamp recordings from laminae I and II neurons. The AMPA/kainate glutamate receptor antagonist CNQX (10-20 microM) regularly abolished DR-EPSCs. In many neurons CNQX also eliminated mEPSCs; however, in a number of cases a proportion of the mEPSCs were resistant to CNQX suggesting that in these instances different mediators or receptors were also involved. Cd2+ (10-50 microM) blocked evoked EPSCs without suppressing mEPSC occurrence. In contrast, Ni2+ (</=100 microM), a low-threshold Ca2+ channel antagonist, markedly decreased mEPSC frequency while leaving evoked monosynaptic EPSCs little changed. Selective organic antagonists of high-threshold (HVA) Ca2+ channels, nimodipine, omega-Conotoxin GVIA, and Agatoxin IVA partially suppressed DR-EPSCs, however, they had little or no effect on mEPSC frequency. La3+ and mibefradil, agents interfering with low-threshold Ca2+ channels, regularly decreased mEPSC frequency with little effect on fast-evoked EPSCs. Increased [K+]o (5-10 mM) in the superfusion, producing modest depolarizations, consistently increased mEPSC frequency; an increase suppressed by mibefradil but not by HVA Ca2+ channel antagonists. Together these observations indicate that different Ca2+ channels are important for evoked EPSCs and mEPSCs in spinal laminae I and II and implicate a low-threshold type of Ca2+ channel in generation of mEPSCs.
Figures



References
-
- Bao J, Li J, Perl ER. Different presynaptic Ca2+ channels influence evoked EPSCs and spontaneous, miniature EPSCs in rodent spinal I and II neurones. J Physiol (Lond) 1997;504.P:175P.
-
- Bao J, Li J, Wall B, Perl ER. Low threshold (T type) Ca2+ channels control miniature EPSC frequency in the spinal substantia gelatinosa. Soc Neurosci Abstr. 1995;21:1572.
-
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. - PubMed
-
- Bezprozvanny I, Tsien RW. Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40–5967). Mol Pharmacol. 1995;48:540–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
