Myelin gene expression after experimental contusive spinal cord injury
- PMID: 9786985
- PMCID: PMC6793561
- DOI: 10.1523/JNEUROSCI.18-21-08780.1998
Myelin gene expression after experimental contusive spinal cord injury
Abstract
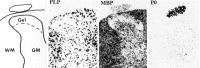
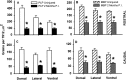
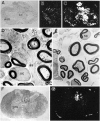
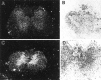
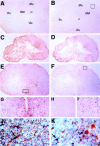
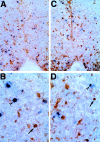
After incomplete traumatic spinal cord injury (SCI), the spared tissue exhibits abnormal myelination that is associated with reduced or blocked axonal conductance. To examine the molecular basis of the abnormal myelination, we used a standardized rat model of incomplete SCI and compared normal uninjured tissue with that after contusion injury. We evaluated expression of mRNA for myelin proteins using in situ hybridization with oligonucleotide probes to proteolipid protein (PLP), the major protein in central myelin; myelin basic protein (MBP), a major component of central myelin and a minor component of peripheral myelin; and protein zero (P0), the major structural protein of peripheral myelin, as well as myelin transcription factor 1 (MYT1). We found reduced expression of PLP and MBP chronically after SCI in the dorsal, lateral, and ventral white matter both rostral and caudal to the injury epicenter. Detailed studies of PLP at 2 months after injury indicated that the density of expressing cells was normal but mRNA per cell was reduced. In addition, P0, normally restricted to the peripheral nervous system, was expressed both at the epicenter and in lesioned areas at least 4 mm rostral and caudal to it. Thus, after SCI, abnormal myelination of residual axons may be caused, at least in part, by changes in the transcriptional regulation of genes for myelin proteins and by altered distribution of myelin-producing cells. In addition, the expression of MYT1 mRNA and protein seemed to be upregulated after SCI in a pattern suggesting the presence of undifferentiated progenitor cells in the chronically injured cord.
Figures




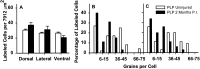
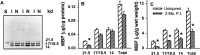

References
-
- Armstrong RC, Kim JG, Hudson LD. Expression of myelin transcription factor I (MyTI), a “zinc-finger” DNA-binding protein, in developing oligodendrocytes. Glia. 1995;14:303–321. - PubMed
-
- Armstrong RC, Migneault A, Shegog ML, Kim JG, Hudson LD, Hessler RB. High-grade human brain tumors exhibit increased expression of myelin transcription factor 1 (MYT1), a zinc finger DNA-binding protein. J Neuropathol Exp Neurol. 1997;56:772–781. - PubMed
-
- Balentine JD. Pathology of experimental spinal cord trauma. II. Ultrastructure of axons and myelin. Lab Invest. 1978;39:254–266. - PubMed
-
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM. Expression of the APC tumor suppressor protein in oligodendroglia. Glia. 1996;17:169–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
