Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation
- PMID: 9786988
- PMCID: PMC6793547
- DOI: 10.1523/JNEUROSCI.18-21-08814.1998
Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation
Abstract
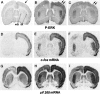
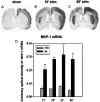
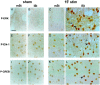
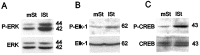
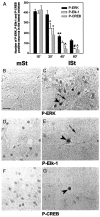
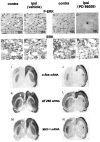
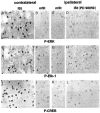
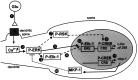
Activity-dependent changes in neuronal structure and synaptic remodeling depend critically on gene regulation. In an attempt to understand how glutamate receptor stimulation at the membrane leads to gene regulation in the nucleus, we traced intracellular signaling pathways targeting DNA regulatory elements of immediate early genes (IEGs). For this purpose we used an in vivo electrical stimulation of the glutamatergic corticostriatal pathway. We show that a transient activation of extracellular signal-regulated kinase (ERK) proteins (detected by immunocytochemistry with an anti-active antibody) is spatially coincident with the onset of IEG induction [c-fos, zif 268, and map kinase phosphatase-1 (MKP-1) detected by in situ hybridization] in the striatum, bilaterally. Both Elk-1 and CREB transcription factors (targeting SRE and CRE DNA regulatory elements, respectively) were hyperphosphorylated in register with ERK activation and IEG mRNA induction. However, their hyperphosphorylation occurred in different subcellular compartments: the cytoplasm and the nucleus for Elk-1 and the nucleus for CREB. The role of the ERK signaling cascade in gene regulation was confirmed after intrastriatal and unilateral injection of the specific ERK inhibitor PD 98059, which completely abolished c-fos, zif 268, and MKP-1 mRNA induction in the injected side. Of interest, both Elk-1 and CREB hyperphosphorylation also was impaired after PD 98059 injection. Thus two different ERK modules, one depending on the cytoplasmic activation of Elk-1 and the other one depending on the nuclear activation of CREB, control IEG transcriptional regulation in our model. Our findings provide significant insights into intracellular mechanisms underlying synaptic plasticity in the striatum.
Figures

References
-
- Abo V, Deniau JM, Rogard M, Besson MJ. Involvement of the NMDA receptors in the induction of Fos expression in basal ganglia following electrical stimulation of the motor cortex. Eur J Neurosci [suppl] 1994;7:101.
-
- Alessi DR, Smythe C, Keyse SM. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene. 1993;8:2015–2020. - PubMed
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Bading H, Greenberg ME. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991;253:912–914. - PubMed
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct signaling pathways. Science. 1993;260:181–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
