Spike-and-wave oscillations based on the properties of GABAB receptors
- PMID: 9787013
- PMCID: PMC6793559
- DOI: 10.1523/JNEUROSCI.18-21-09099.1998
Spike-and-wave oscillations based on the properties of GABAB receptors
Abstract
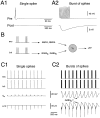
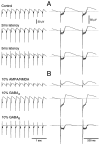
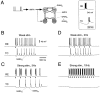
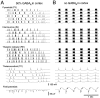
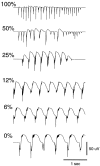
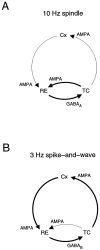
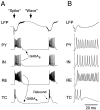
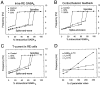
Neocortical and thalamic neurons are involved in the genesis of generalized spike-and-wave (SW) epileptic seizures. The cellular mechanism of SW involves complex interactions between intrinsic neuronal firing properties and multiple types of synaptic receptors, but because of the complexity of these interactions the exact details of this mechanism are unclear. In this paper these types of interactions were investigated by using biophysical models of thalamic and cortical neurons. It is shown first that, because of the particular activation properties of GABAB receptor-mediated responses, simulated field potentials can display SW waveforms if cortical pyramidal cells and interneurons generate prolonged discharges in synchrony, without any other assumptions. Here the "spike" component coincided with the synchronous firing, whereas the "wave" component was generated mostly by slow GABAB-mediated K+ currents. Second, the model suggests that intact thalamic circuits can be forced into a approximately 3 Hz oscillatory mode by corticothalamic feedback. Here again, this property was attributable to the characteristics of GABAB-mediated inhibition. Third, in the thalamocortical system this property can lead to generalized approximately 3 Hz oscillations with SW field potentials. The oscillation consisted of a synchronous prolonged firing in all cell types, interleaved with a approximately 300 msec period of neuronal silence, similar to experimental observations during SW seizures. This model suggests that SW oscillations can arise from thalamocortical loops in which the corticothalamic feedback indirectly evokes GABAB-mediated inhibition in the thalamus. This mechanism is shown to be consistent with a number of different experimental models, and experiments are suggested to test its consistency.
Figures

References
-
- Avoli M, Gloor P. The effect of transient functional depression of the thalamus on spindles and bilateral synchronous epileptic discharges of feline generalized penicillin epilepsy. Epilepsia. 1981;22:443–452. - PubMed
-
- Avoli M, Gloor P. Role of the thalamus in generalized penicillin epilepsy: observations on decorticated cats. Exp Neurol. 1982;77:386–402. - PubMed
-
- Avoli M, Gloor P, Kostopoulos G, Gotman J. An analysis of penicillin-induced generalized spike and wave discharges using simultaneous recordings of cortical and thalamic single neurons. J Neurophysiol. 1983;50:819–837. - PubMed
-
- Buzsáki G, Smith A, Berger S, Fisher LJ, Gage FH. Petit mal epilepsy and parkinsonian tremor: hypothesis of a common pacemaker. Neuroscience. 1990;36:1–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
