Culture of mouse spermatogonial stem cells
- PMID: 9787472
- PMCID: PMC5507671
- DOI: 10.1016/s0040-8166(98)80053-0
Culture of mouse spermatogonial stem cells
Abstract
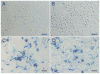
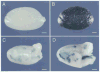
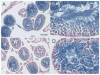
Spermatogenesis occurs within the seminiferous tubules of mammals by a complex process that is highly organized, extremely efficient and very productive. At the foundation of this process is the spermatogonial stem cell that is capable of both self-renewal and production of progeny cells, which undergo differentiation over a period of weeks to months in order to generate mature spermatozoa. It had been thought that germ cells survive only a brief period in culture, generally less than a few weeks. However, an accurate assessment of the presence of spermatogonial stem cells in any cell population has only recently become possible with development of the spermatogonial transplantation technique. Using this technique, we have demonstrated that mouse spermatogonial stem cells can be maintained in culture for approximately 4 months and will generate spermatogenesis following transplantation to the seminiferous tubules of an appropriate recipient. Extensive areas of cultured donor cell-derived spermatogenesis are generated in the host, and production of mature spermatozoa occurs. Cultivation of the testis cells on STO feeders is beneficial to stem cell survival. These results provide the first step in establishing a system that will permit spermatogonial stem cells to be cultivated and their number increased in vitro to allow for genetic modification before transplantation to a recipient testis.
Figures

References
-
- Bardin CW, Gunsalus GL, Cheng CY. The cell biology of the Sertoli cell. In: Desjardins C, Ewing LL, editors. Cell and molecular biology of the testis. Oxford University Press; New York: 1993. pp. 189–219.
-
- Bellvé AR. Purification, culture, and fractionation of spermatogenic cells. In: Wassarman PM, DePamphilis ML, editors. Methods in enzymology. Vol. 225. Academic Press; New York: 1993. pp. 84–113. - PubMed
-
- Brinster RL. Cultivation of the mammalian egg. In: Rothblat G, Cristofal V, editors. Growth, nutrition and metabolism of cells in culture. Vol. 2. Academic Press; New York: 1972. pp. 251–286.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

