Folding in vivo of a newly translated yeast cytosolic enzyme is mediated by the SSA class of cytosolic yeast Hsp70 proteins
- PMID: 9789005
- PMCID: PMC23633
- DOI: 10.1073/pnas.95.22.12860
Folding in vivo of a newly translated yeast cytosolic enzyme is mediated by the SSA class of cytosolic yeast Hsp70 proteins
Abstract
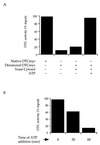
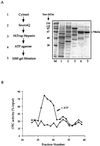
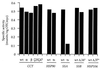
The nature of chaperone action in the eukaryotic cytosol that assists newly translated cytosolic proteins to reach the native state has remained poorly defined. Actin, tubulin, and Galpha transducin are assisted by the cytosolic chaperonin, CCT, but many other proteins, for example, ornithine transcarbamoylase (OTC), a cytosolic homotrimeric enzyme of yeast, do not require CCT action. Here, we observe that yeast cytosolic OTC is assisted to its native state by the SSA class of yeast cytosolic Hsp70 proteins. In vitro, refolding of OTC diluted from denaturant was assisted by crude yeast cytosol and ATP and found to be directed by SSA1/2. In vivo, when OTC was induced in a temperature-sensitive SSA-deficient strain, it exhibited reduced specific activity, and nonnative subunits were detected in the soluble fraction. These findings indicate that, in vivo, the Hsp70 system assists in folding at least some newly translated cytosolic enzymes, most likely functioning in a posttranslational manner.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

