Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis
- PMID: 9789033
- PMCID: PMC23692
- DOI: 10.1073/pnas.95.22.13018
Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis
Abstract
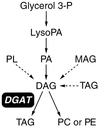
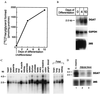
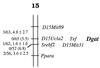
Triacylglycerols are quantitatively the most important storage form of energy for eukaryotic cells. Acyl CoA:diacylglycerol acyltransferase (DGAT, EC 2.3.1.20) catalyzes the terminal and only committed step in triacylglycerol synthesis, by using diacylglycerol and fatty acyl CoA as substrates. DGAT plays a fundamental role in the metabolism of cellular diacylglycerol and is important in higher eukaryotes for physiologic processes involving triacylglycerol metabolism such as intestinal fat absorption, lipoprotein assembly, adipose tissue formation, and lactation. DGAT is an integral membrane protein that has never been purified to homogeneity, nor has its gene been cloned. We identified an expressed sequence tag clone that shared regions of similarity with acyl CoA:cholesterol acyltransferase, an enzyme that also uses fatty acyl CoA as a substrate. Expression of a mouse cDNA for this expressed sequence tag in insect cells resulted in high levels of DGAT activity in cell membranes. No other acyltransferase activity was detected when a variety of substrates, including cholesterol, were used as acyl acceptors. The gene was expressed in all tissues examined; during differentiation of NIH 3T3-L1 cells into adipocytes, its expression increased markedly in parallel with increases in DGAT activity. The identification of this cDNA encoding a DGAT will greatly facilitate studies of cellular glycerolipid metabolism and its regulation.
Figures


References
-
- Bell R M, Coleman R A. Annu Rev Biochem. 1980;49:459–487. - PubMed
-
- Lehner R, Kuksis A. Prog Lipid Res. 1996;35:169–201. - PubMed
-
- Brindley D N. In: Biochemistry of Lipids, Lipoproteins and Membranes. Vance D E, Vance J E, editors. Amsterdam: Elsevier; 1991. pp. 171–203.
-
- Nishizuka Y. Science. 1992;258:607–614. - PubMed
-
- Haagsman H P, Van Golde L M G. Arch Biochem Biophys. 1981;208:395–402. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

